Application of Artemisinin Compounds in the Preparation of Medicines for Treating Neuropathic Pain and/or Complications
An artemisinin and compound technology, which is applied in the application field of artemisinin compounds in the preparation of medicines for the treatment of neuropathic pain and/or complications, can solve the problem of increasing ataxia, confusion, abnormal thinking, and blurred vision. Difficulty in paying attention to ataxia, association with neuropathic pain, inability to infer drug efficacy, etc., to achieve the effect of drug safety, low drug resistance, and low toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0055] Experimental example 1: Experimental preparation, model construction and detection method
[0056] 1. Experimental animals
[0057] Clean-grade 8-week-old adult male C57 / BL6 mice, weighing 24-26 g, were provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., animal certificate number SYXK-2010-0034.
[0058] 2. Experimental grouping
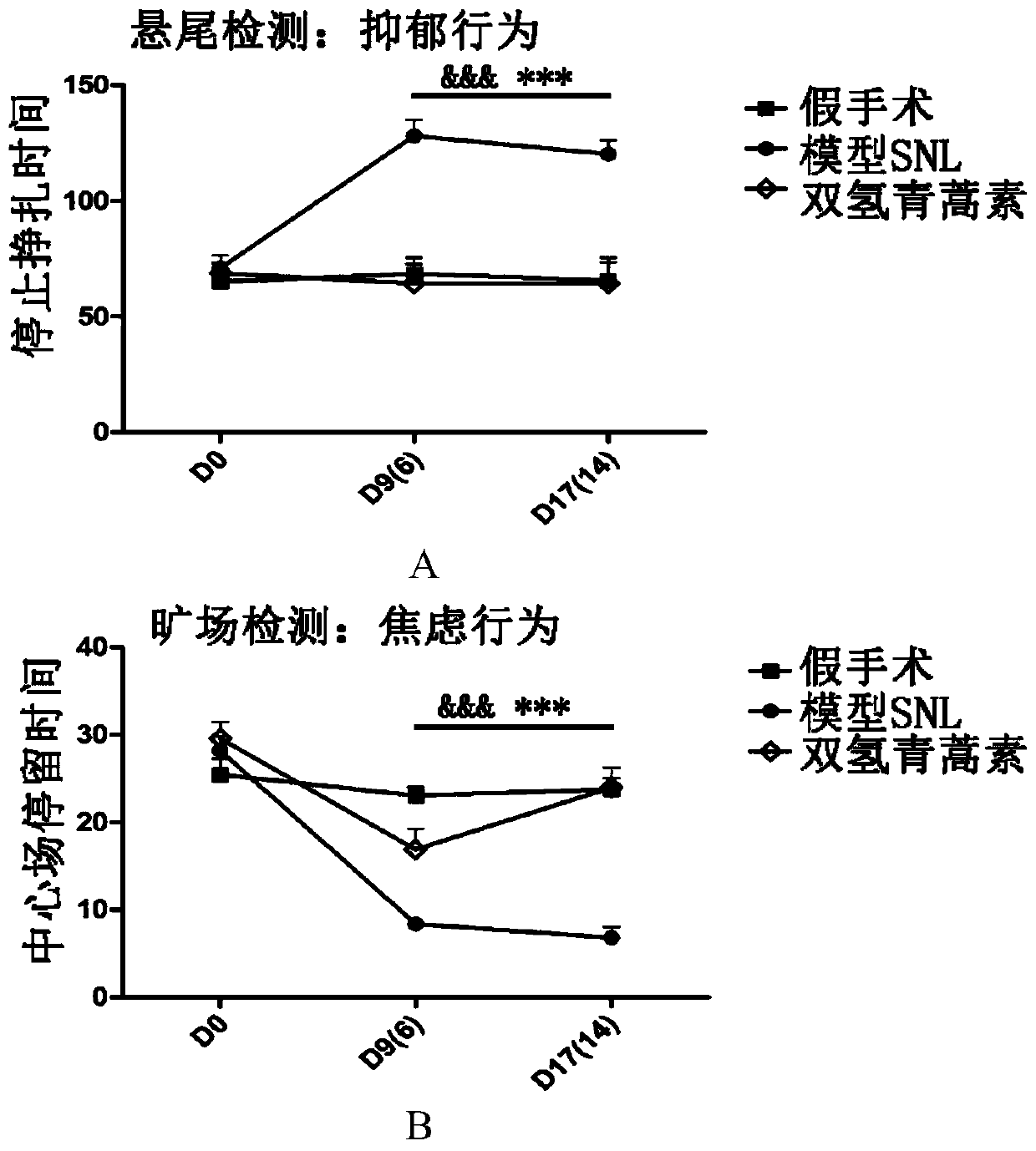
[0059] After adapting to the environment for 4 days, the mice were weighed and subjected to open-field testing, forced swimming testing and tail-suspension testing. The mice whose body weight was less than 23 grams, the time spent in the center field of the open-field test was less than 15 seconds, and the forced swimming time of more than 10 seconds were excluded. 160 seconds, or individuals whose immobility time is beyond 50-100 seconds by tail suspension, and then randomly divided into sham operation group, model SNL group, and medication group, each group in each parallel experiment is more than 12.
[0060] 3. Es...
experiment example 2
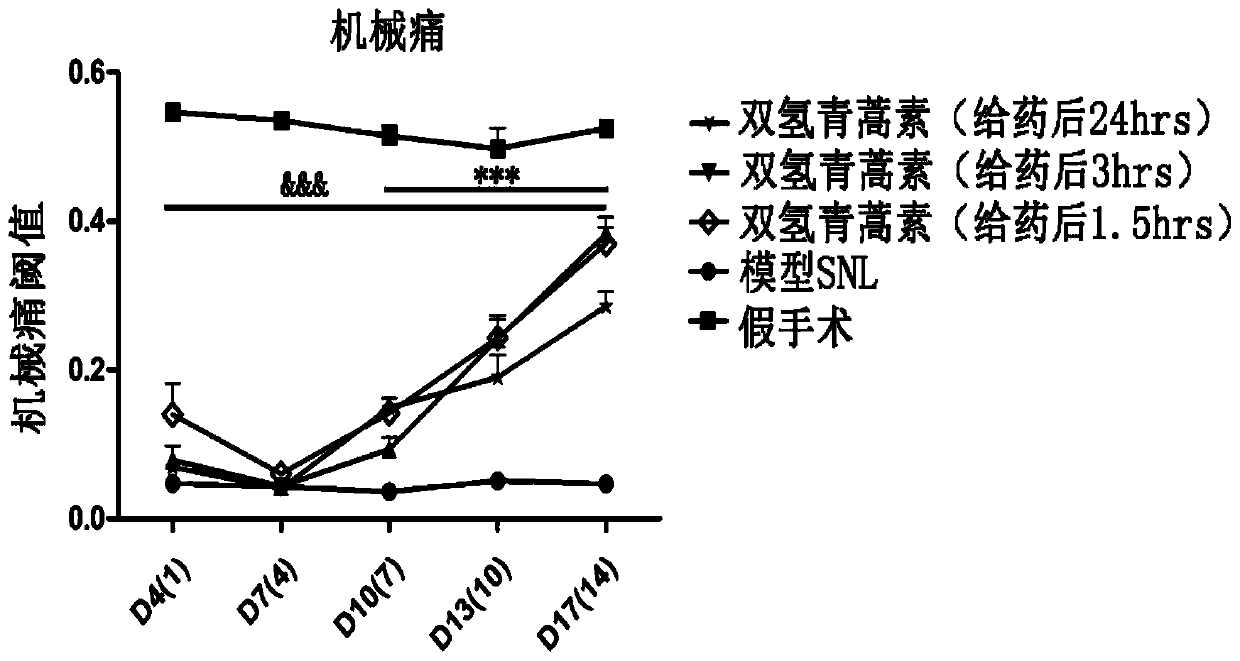
[0075] Experimental Example 2: Therapeutic Effects of Dihydroartemisinin on Mechanical Allodynia, Depression and Anxiety in Neuropathic Pain
[0076] 1. Drug preparation and dosing regimen
[0077] The day of modeling / sham operation is recorded as D0. In the drug group, the ratio of 40 mg / kg (artemisinin analogue or positive drug pregabalin / mouse body weight) was administered orally, and the volume of the drug solution was 150 μL; dihydroartemisinin or pregabalin was suspended in 150 μL beforehand The medicine solution was made from the oil; the sham operation group and the model SNL group used the same volume of edible oil instead of the medicine solution, and the administration time and route were the same as those of the drug treatment group.
[0078] In the early administration experiment, the drug was given by intragastric administration from the 4th day, which was recorded as D4(1). The intragastric administration was carried out at 5:00 p.m. every day for 14 consecuti...
experiment example 3
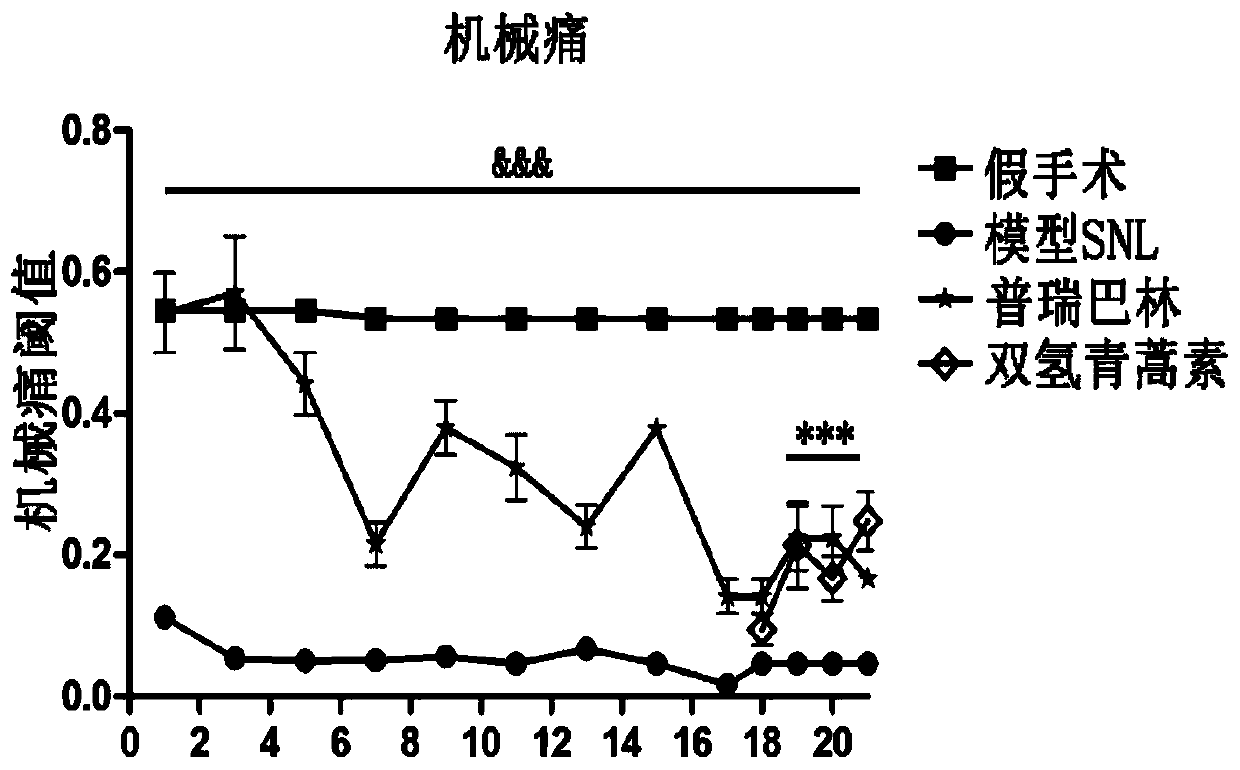
[0096] Experimental Example 3: Therapeutic Effects of Artesunate on Mechanical Allodynia, Depression and Anxiety in Neuropathic Pain
[0097] 1. Drug preparation
[0098] Artesunate was pre-suspended in 150 μL of 5% medical sodium bicarbonate injection to make a drug solution; the sham operation group and the model SNL group used 5% medical sodium bicarbonate injection as the solvent carrier.
[0099] 2. Observation indicators
[0100] The mechanical pain threshold was detected on D4, D7, D10, and D13 after modeling, and the detection time points were 1.5 hours, 3 hours, and 24 hours after intragastric administration.
[0101] Tail suspension test and open field test were carried out on D9 and D17 days after modeling, respectively.
[0102] 3. Experimental results
[0103] 3.1 Artesunate can increase mechanical pain threshold and reduce pain sensitivity
[0104] The test results of the influence of artesunate on the mechanical pain threshold are as follows: Figure 5 It s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com