Method for purifying chondroitinase ABC
A chondroitinase, heparin technology, applied in biochemical equipment and methods, enzymes, enzymes and other directions, can solve difficulties and other problems, and achieve the effect of high recovery rate and simple recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In the present invention, the identification of molecules that have a similar structure to the substrates of ChABC but are inert to enzymatic degradation is the first critical part of the invention. To test for polysaccharides that can actually bind to the active site of ChABC, a relative enzyme inhibition assay was performed on selected polysaccharides:
[0050] 490 [mu]L of substrate solution containing 0.2% chondroitin sulfate C, 0.2% selected polysaccharide and 50 mM pH 8.0 Tris HCl buffer was incubated at 37[deg.] C. for at least 15 minutes. 10 μL of ChABC with an activity of about 100 U / L was incubated alone for at least 3 minutes at 37°C. After incubation, mix the two solutions gently. The mixture was then incubated at 37°C for 20 minutes for enzymatic degradation of chondroitin sulfate. After 20 min, the reaction was terminated by heat inactivating ChABC in a 100 °C water bath for 2 min. Blanks were performed separately in the same manner except that the mixt...
Embodiment 2
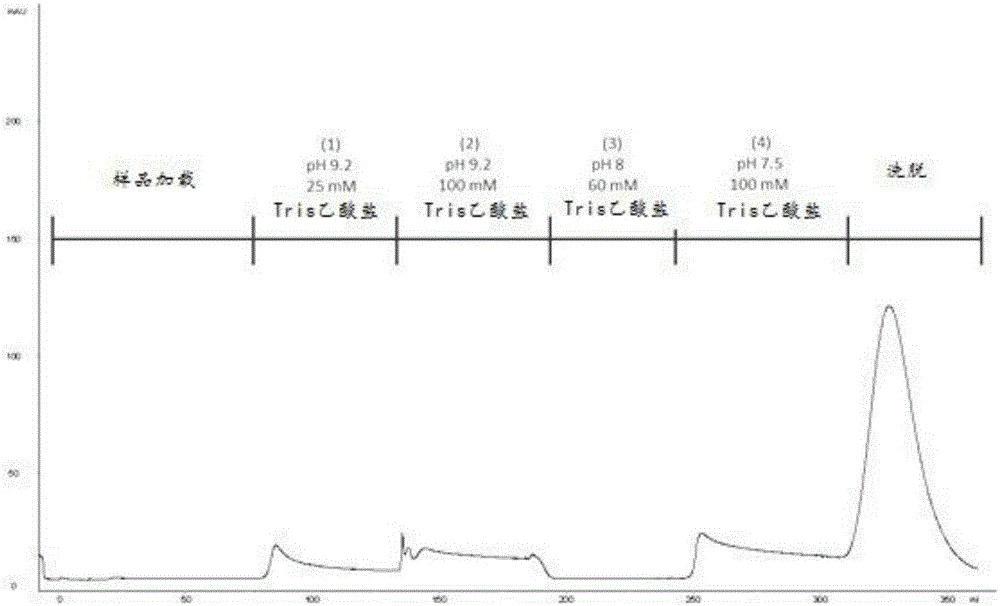
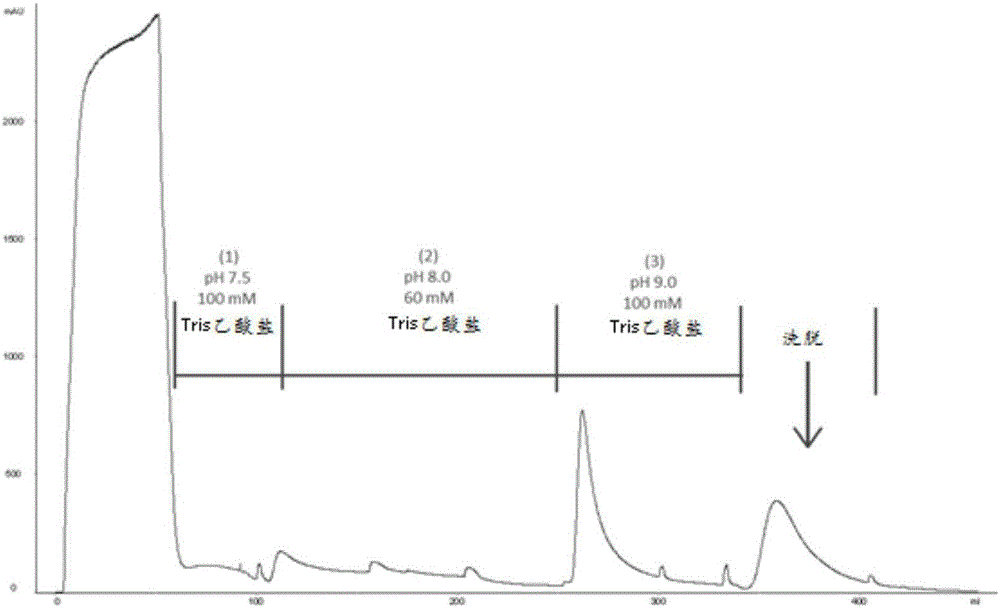
[0070] To test whether ChABC specifically interacts with immobilized heparin, Affi Columns of heparin gel. The column was pre-equilibrated with 25 mM Tris-acetate (pH 7.3) prior to loading samples onto the column. ChABC was dissolved in 25 mM Tris-acetate (pH 7.5) and loaded onto the column. With (1) 25mM Tris-acetate (pH9.2), (2) 100mM Tris-acetate (pH9.2), (3) 60mM Tris-acetate (pH8) and (4) 100mM Tris-acetate The column was washed with hydrochloric acid (pH 7.5). None of these buffers could elute ChABC from the column. Whereas ChABC could be eluted from the column when a linear gradient of 0-0.3M NaCl was applied. Chromatograms such as figure 1 shown.
Embodiment 3
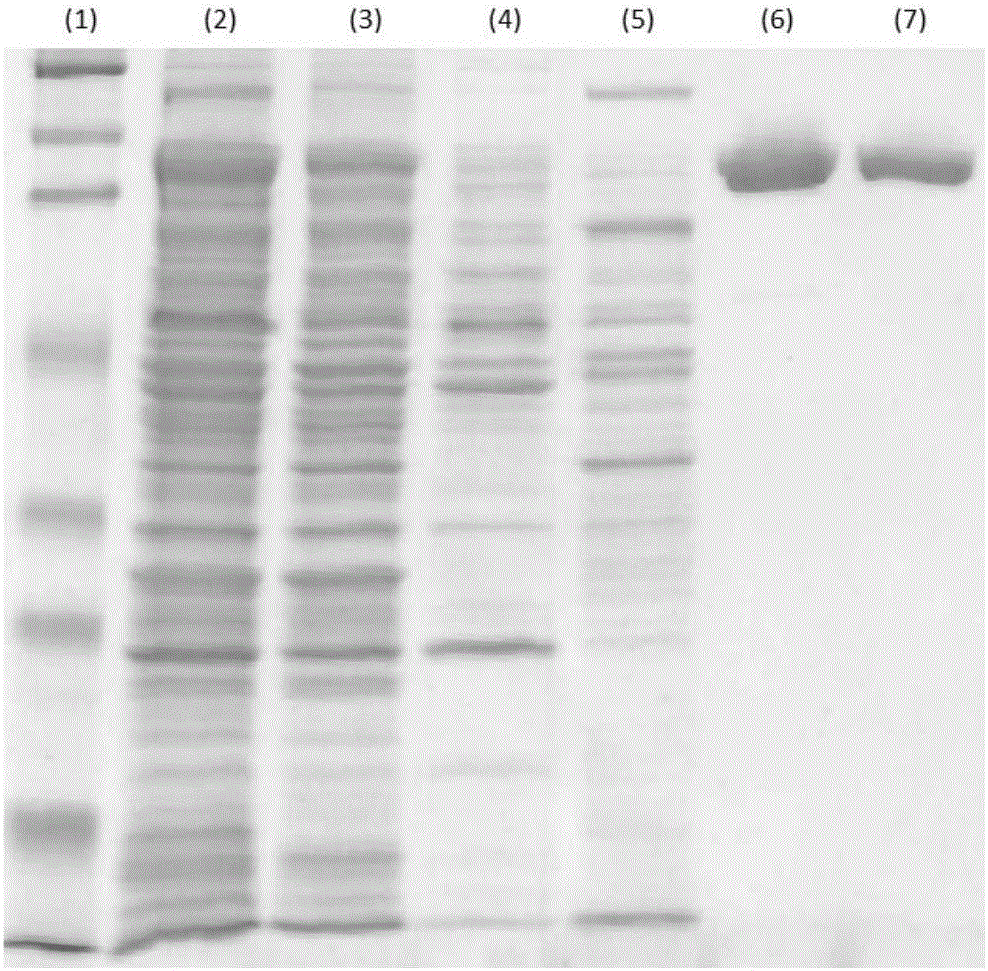
[0072] Using the genome of Proteus vulgaris as a template, the gene encoding chondroitinase ABC (SEQ ID NO: 1) was cloned by PCR, and the product was connected to the pET3a plasmid by T4DNA ligase, and then the recombinant plasmid was introduced into the E. coli strain by electroporation BL21(DE3). The transformed E. coli were inoculated on LB agar plates containing 50 μg / ml ampicillin to select successfully transformed colonies. Select a single colony, then inoculate it into LB medium containing 50 μg / ml ampicillin at 37 °C for 18 hours, and add the culture to sterile LB medium containing 50 μg / ml ampicillin at a ratio of 1:100 . The medium was further incubated at 37°C to OD600=0.8. Addition of 1 mM IPTG induced chondroitinase production, followed by further incubation for 4 hours. Harvest by centrifugation and store at -80 °C.
[0073] 10 grams of recombinant E. coli cells were resuspended in 25 mM Tris HCl (pH 7.0) and lysed by sonication for 5 minutes. Cell debris wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com