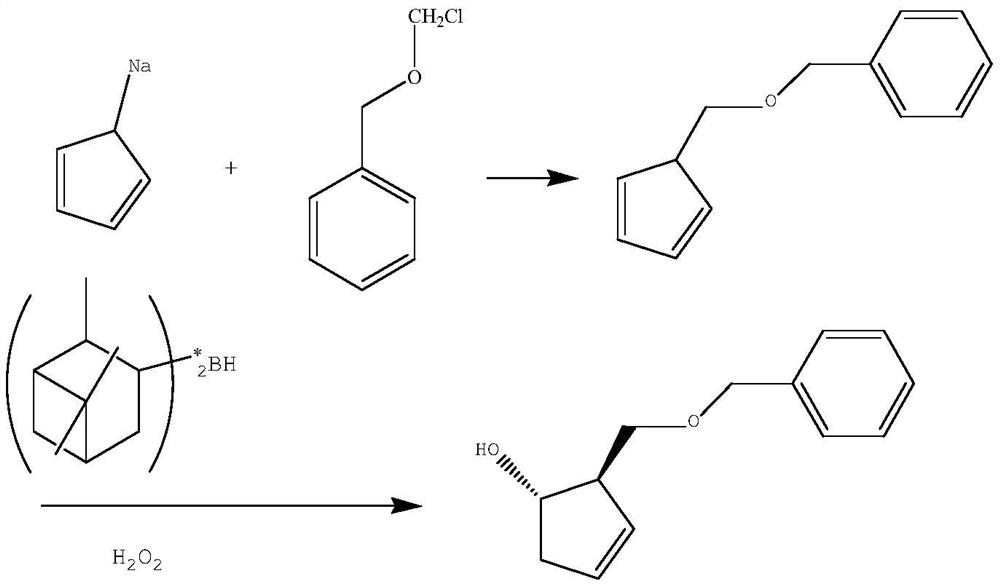
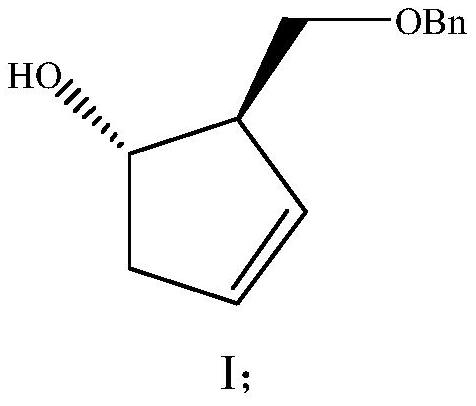
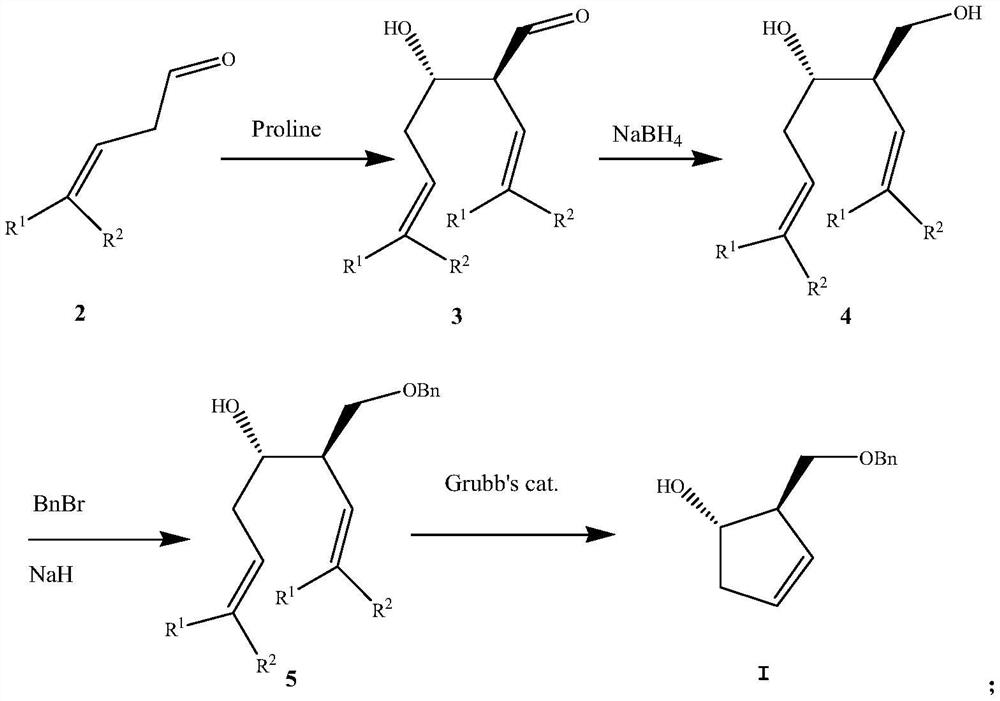
A kind of preparation method of pharmaceutical intermediate optically pure cyclopentenol
A technology of pure cyclopentenol and intermediates, applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of expensive reagents and high requirements for reaction conditions, and achieve low price, simple operation, and high reaction efficiency. The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 (2R, 3S)-3-hydroxyl-2-vinylhex-5-ene-1-aldehyde
[0036] (S)-proline (14.9 mg, 0.13 mmol) was added to a stirred solution of 3-butenal (150 mg) in 1 ml of tetrahydrofuran, stirred at 25°C for 20 h, and the reaction solution was concentrated with petroleum The mixture of ether and ethyl acetate was separated by liquid column chromatography to obtain 100 mg of (2R,3S)-3-hydroxy-2-vinylhex-5-en-1-al. Yield 67%, 1 H NMR (DMSO-d 6 )δ:1.95(brs,1H),2.11(m,2H),3.11(m,1H),3.51(m,1H),4.97(d,1H),5.03(d,1H),5.17(d,1H ), 5.19(d,1H), 5.70(dd,1H), 5.97(dd,1H), 9.72(s,1H).
Embodiment 2
[0037] The preparation of embodiment 2 (2R, 3S)-3-hydroxyl-2-vinylhex-5-en-1-alcohol
[0038] 2 mmol (222 mg) of calcium chloride, 1 mmol (140 mg) (2R, 3S)-3-hydroxyl-2-vinylhex-5-en-1-aldehyde were added to a 50-mL one-necked flask, Add 5 ml of methanol and stir at 25°C for 30 minutes. 1.2 mmol (50 mg) of sodium borohydride was dissolved in a solution prepared by 1 ml of methanol and 1 ml of 1% sodium hydroxide, and the sodium borohydride solution was added dropwise under ice-cooling and stirring. After the dropwise addition, the stirring reaction was continued at 25° C. for 30 minutes, and then 1 ml of 4M hydrochloric acid solution was added to decompose the solid that appeared. Most of the methanol was removed under reduced pressure, cooled to 25°C, 10 ml of ice water was added, extracted three times with dichloromethane, the organic phases were combined, dried and concentrated, and separated by column chromatography to obtain (2R,3S)-3-hydroxy-2- Vinylhex-5-en-1-ol 120 m...
Embodiment 3
[0039] The preparation of embodiment 3 (3R, 4S)-3-benzyloxymethylhepta-1,6-dien-4-ol
[0040] 142 mg of (2R,3S)-3-hydroxy-2-vinylhex-5-en-1-ol (1.0 mmol) was added to 200 mg of sodium hydride in 2 ml of DMF under cooling at 0 °C, then 342 mg (2 mmol) of benzyl bromide was added with stirring. After the addition, continue to stir at 25°C for 30 minutes. After no raw material spots are detected by thin-plate chromatography, the reaction solution is poured into 50 grams of ice, extracted three times with dichloromethane, and the extracts are combined, dried, concentrated, and purified by column chromatography. 205 mg of (3R,4S)-3-benzyloxymethylhept-1,6-dien-4-ol was obtained with a yield of 88%. 1 H NMR (DMSO-d 6)δ:2.00(brs,1H),2.11(m,2H),2.56(m,1H),3.28(m,1H),3.37(m,2H),4.63(m,2H),4.97(m,2H ), 5.02(m,2H), 5.70(m,2H), 7.19-7.40(m,5H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com