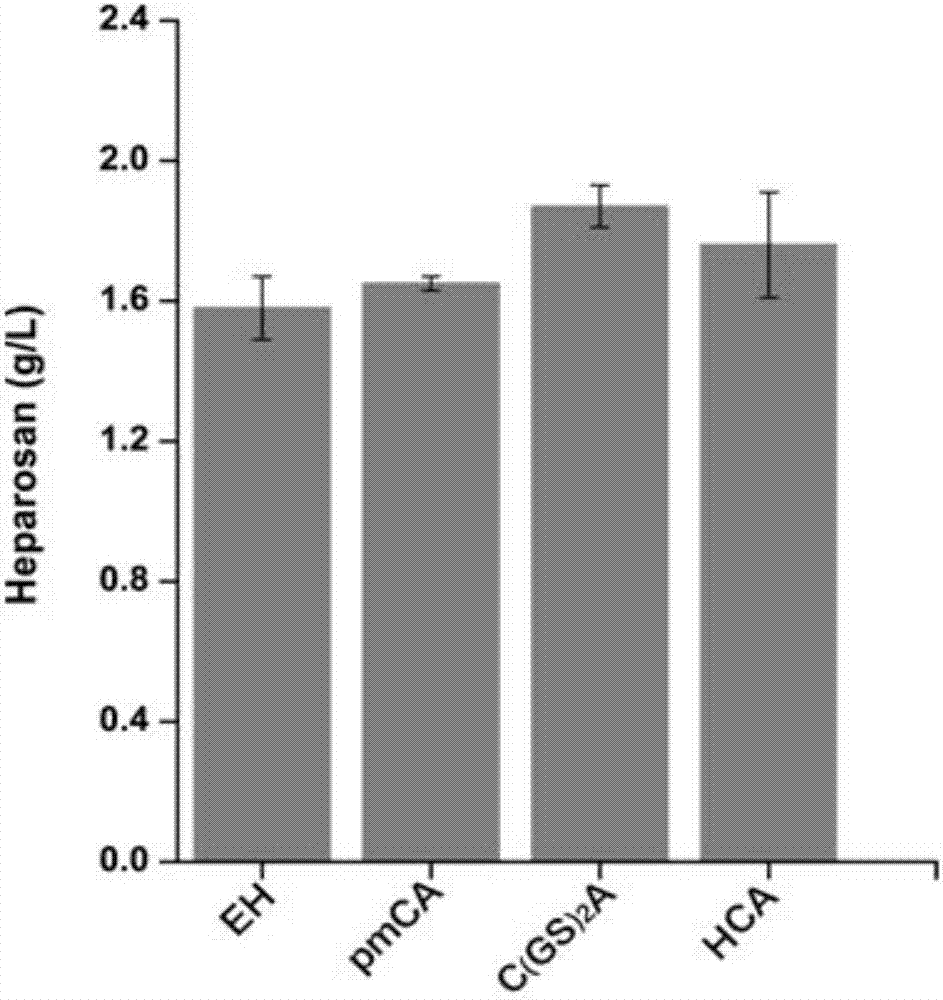
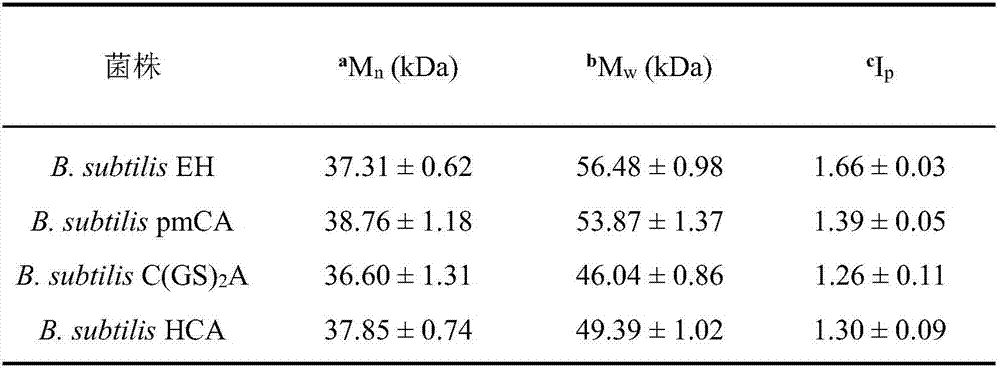
Heparin precursor synthase and application thereof
A heparin precursor and sequence technology, which is applied to heparin precursor synthase and its application field, can solve the problems of large molecular weight, disadvantage, influence polymerization rate and efficiency, etc., and achieve the effect of reducing molecular weight and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Construction of Escherichia coli K5 heparin precursor synthase KfiC and KfiA co-expression recombinant plasmid
[0034]α-glycosyltransferase encoding gene kfiA and β-glycosyltransferase encoding gene kfiC are derived from Escherichia coli K5 (E.coli O10:K5:H4, E.coli K5), E.coli K5 strain was inoculated in 5mL LB liquid Medium, cultivated at 37°C 200rpm for 16h. The bacteria were collected, and the genomic DNA of the E.coli K5 strain was extracted using a bacterial genome extraction kit.
[0035] Primers KfiA-F1 / KfiA-R1, KfiC-F1 / KfiC-R1 were designed respectively, and the extracted genomic DNA was used as a template to amplify the target gene using standard PCR amplification system and procedures.
[0036] Primer sequence information: 5'-3' direction
[0037] KfiA-F1: GGTAAGAGAGGAATGTACACATGATTGTTGCAAATATGTC
[0038] KfiA-R1: CCGCTCGAG TTACCCTTCCACATTATACA
[0039] KfiC-F1: CGGGGTACC ATGAACGCAGAATATATAAATTTAG
[0040] KfiC-R1:GTGTACATTCCTCTCTACCCTATTG...
Embodiment 2
[0042] Example 2 Escherichia coli K5 heparin precursor synthases KfiC and KfiA through a flexible linker (GGGGS) 2 Connection construction
[0043] Using the above-mentioned recombinant plasmid pP43NMK-kfiC-RBS-kfiA as a template, design primers KfiA-F2 / KfiA-R1, KfiC-F1 / KfiC-R2 respectively, and use standard PCR amplification system and program to amplify and obtain the target gene.
[0044] Primer sequence information: 5'-3' direction
[0045] KfiA-F2: GGTGGCGGTGGCTCGGGCGGTGGTGGGTCGATGATTGTTGCAAATATGTC
[0046] KfiC-R2:CGACCCCACCACCGCCCGAGCCACCGCCACCTTGTTCAATTATTTCCTGATA
[0047] KpnI restriction enzyme sites and (GGGGS) were introduced at both ends of the upstream and downstream primers of kfiC, respectively. 2 linker sequence, and remove the stop codon of kfiC. Introduce (GGGGS) at both ends of the upstream and downstream primers of kfiA 2 linker sequence and XhoI restriction enzyme site. The obtained kfiC and kfiA fragments were amplified by PCR (GGGGS) 2 In the ove...
Embodiment 3
[0048] Example 3 Construction of fusion of Escherichia coli K5 heparin precursor synthase KfiC and KfiA
[0049] Using the recombinant plasmid pP43NMK-kfiC-RBS-kfiA as a template, primers KfiA-F3 / KfiA-R1 and KfiC-F1 / KfiC-R3 were designed respectively, and the target gene was amplified using standard PCR amplification system and procedures.
[0050] Primer sequence information: 5'-3' direction
[0051] KfiA-F3: AGATGTATCAGGAATAATTGAACAAATTGTTGCAAATATGTCATC
[0052] KfiC-R3:TTGTTCAATTTATTCCTGATA
[0053] The KpnI restriction site and the 20 bp sequence of kfiA after the start codon ATG (excluding ATG) were respectively introduced at both ends of the upstream and downstream primers of kfiC, and the stop codon of kfiC was removed. An XhoI restriction enzyme site was introduced at the 5' end of the kfiA downstream primer. After PCR amplification of the obtained kfiC and kfiA fragments, the two fragments were fused by 20bp overlapping PCR to obtain the fragment KpnI-kfiC-kfiA-Xho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com