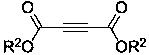Synthesis method of benzothiophene compound
A benzothiophene and synthesis method technology, applied in the field of organic compounds and synthesis, can solve the problems of large substrate limitations, increased production costs, and large environmental pollution, and achieve simple processing, high use safety, and low environmental pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
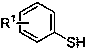
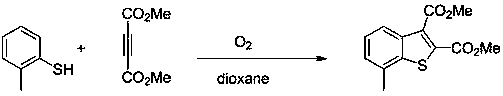
[0023] Example 1: Synthesis of dimethyl 5-methylbenzothiophene-2,3-dicarboxylate (compound A)
[0024] Magnets were added to a 25ml round bottom flask, followed by the compound p-Cresol (0.5mmol) and compound Dimethyl butynedioate (1mmol), 1,2-dioxane (10ml) were added to a round bottom flask at 80 o Stir at C for 4h, remove the solvent by distillation under reduced pressure, and carry out column chromatography separation (silica gel: 200~300 mesh, the eluent is a volume ratio of petroleum ether:ethyl acetate=50:1v / v), and remove the solvent by distillation under reduced pressure. solvent to obtain a white powdery pure product, dimethyl 5-methylbenzothiophene-2,3-dicarboxylate (Compound A), with a yield of 90%. Its synthetic formula is as follows:
[0025]
[0026] The NMR data of Compound A are as follows: 1 H NMR (600 MHz, CDCl 3 ) δ 7.72 (d, J = 8.4 Hz, 1H), 7.70 (s, 1H), 7.31 (d, J = 8.3 Hz, 1H), 4.02 (s, 3H), 3.93 (s, 3H). 13 C NMR (151 MHz, CDCl 3 ) δ 1...
Embodiment 2
[0027] Example 2: Synthesis of Dimethyl Benzothiophene-2,3-dicarboxylate (Compound B)
[0028] Magnets were added to a 25ml round bottom flask, followed by the compound Thiophenol (0.5mmol) and compound Dimethyl butynedioate (1mmol), 1,2-dioxane (10ml) were added to a round bottom flask at 80 o Stir for 2 hours at C, remove the solvent by distillation under reduced pressure, and carry out column chromatography separation (silica gel: 200~300 mesh, the eluent is a volume ratio of petroleum ether:ethyl acetate=50:1v / v), and remove the solvent by distillation under reduced pressure. solvent, the pure product was obtained as white powder, namely dimethyl benzothiophene-2,3-dicarboxylate (compound B), and the yield was 70%. Its synthetic formula is as follows:
[0029]
[0030] The NMR data of Compound B are as follows: 1 H NMR (600 MHz, CDCl 3 ) δ 7.93 (d, J= 7.7 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.50 - 7.43 (m, 2H), 4.02 (s, 3H), 3.94 (s, 3H). 13 CNMR (151 MHz, CDC...
Embodiment 3
[0031] Example 3: Synthesis of dimethyl 7-methylbenzothiophene-2,3-dicarboxylate (compound C)
[0032] Compound Ⅰ is o-methylthiophenol (0.5mol), compound It is dimethyl butynedioate (1mol), and the reaction time is 2h; the synthesis route and separation method are basically the same as in Example 1. The white powdery product obtained was dimethyl 7-methylbenzothiophene-2,3-dicarboxylate (compound C), and the yield was 85%. Its synthetic formula is as follows:
[0033]
[0034] The NMR data of Compound C are as follows: 1 H NMR (600 MHz, CDCl 3 ) δ 7.76 (d, J = 8.2 Hz, 1H), 7.38 (t, J = 7.7 Hz, 1H), 7.28 (d, J = 7.2 Hz, 1H), 4.02 (s, 3H), 3.95 (s,3H), 2.56 (s, 3H). 13 C NMR (151 MHz, CDCl 3 ) δ 165.0, 162.2, 140.7, 136.6, 134.0, 132.4, 132.2, 127.5, 126.1, 122.1, 52.9, 52.8, 20.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com