Chromatography medium as well as preparation method and application thereof
A chromatographic medium and matrix technology, applied in the field of chromatographic medium and its preparation, can solve the problems of limited processing capacity, no effective improvement of adsorption capacity, and advantages of difficult antibodies, and achieves high dynamic binding capacity and non-specific medium. The effect of small anisotropic adsorption and significant adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
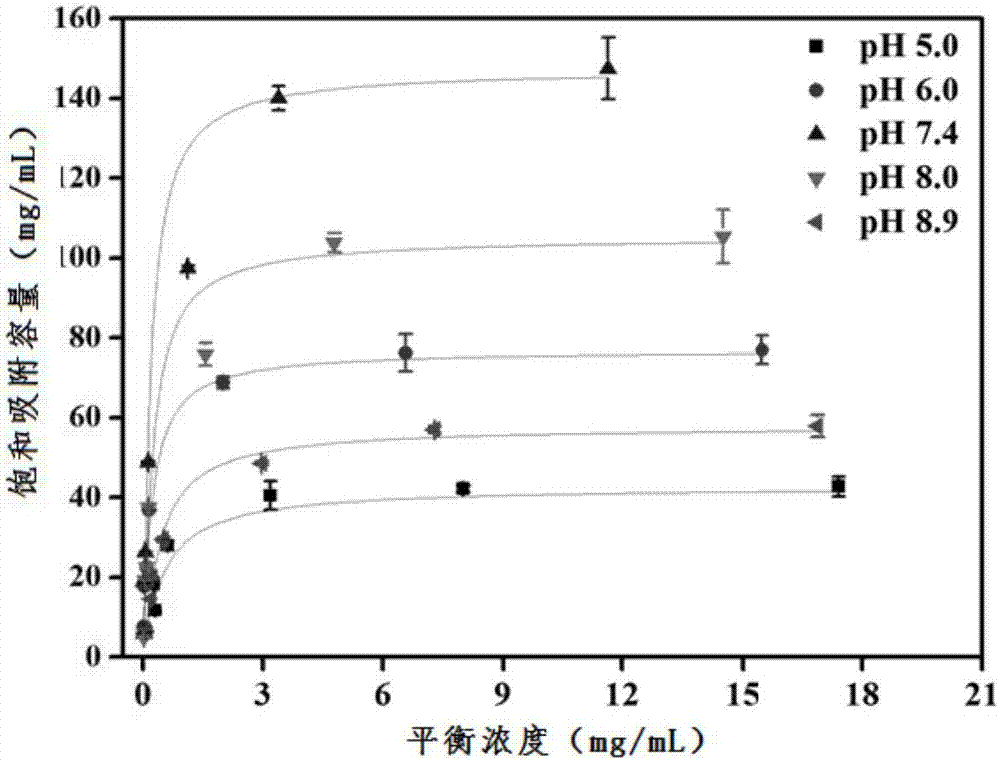
Examples
Embodiment 1
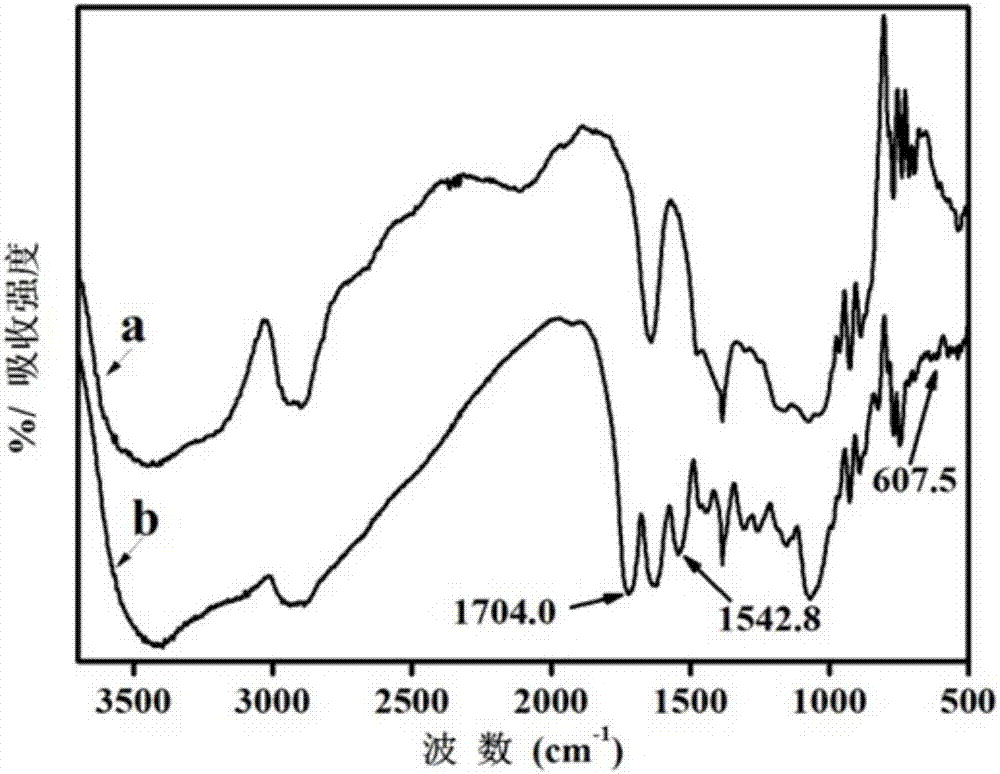
[0057] Example 1 Using 3,3'-diaminodipropylamine as a spacer to couple 5-carboxybenzotriazole
[0058] ⑴Activation
[0059] Take cross-linked agarose gel (GE Healthcare, Sepharose TM CL-6B) 10g, rinse with deionized water several times, then wash with appropriate amount of acetone for 3-5 times, drain and add to 20-40mL acetone solution containing 0.1-1.0g of N,N'-carbonyldiimidazole in a shaker at 25-37°C, 150-200rpm for 2-5 hours, after activation, wash with acetone, and filter with suction to obtain activated cross-linked agarose gel;
[0060] ⑵Grafted spacer arm
[0061] Add the activated cross-linked agarose gel and 1~3g of 3,3'-diaminodipropylamine into 20~40mL acetone solution, and react in a shaker at 30~37℃, 150~200rpm for 5~8 After the reaction, wash with acetone to remove unreacted 3,3'-diaminodipropylamine, and drain to obtain a cross-linked agarose with 3,3'-diaminodipropylamine grafted at the end as a spacer arm gel;
[0062] ⑶Coupling functional ligand
[...
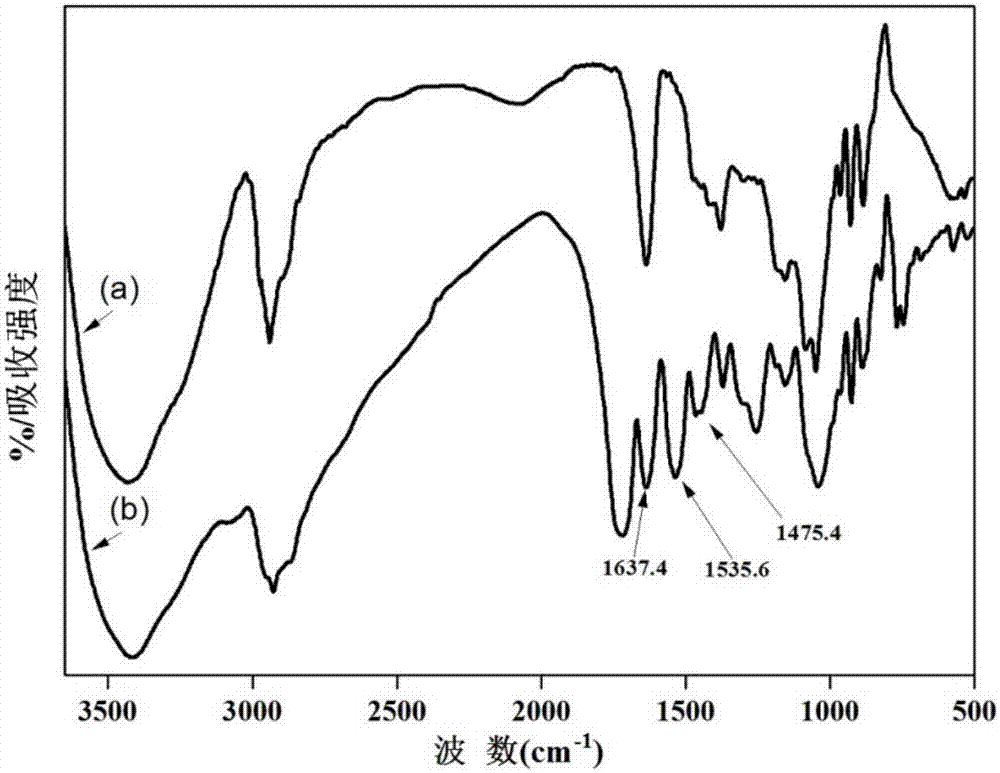
Embodiment 2
[0064] Embodiment 2 utilizes 1,6-hexamethylenediamine as a spacer coupling ligand
[0065] ⑴Activation
[0066] Take cross-linked agarose gel (GE Healthcare, Sepharose TM CL-6B) 10g, rinsed with deionized water several times to remove 20% ethanol, then washed with an appropriate amount of acetone for 3 to 5 times, drained and added to the In 20-40mL acetone solution, activate in a shaker at 25-37°C, 150-200rpm for 2-5 hours, wash with acetone after activation to remove unreacted N,N'-carbonyldiimidazole, and obtain activated The cross-linked agarose gel;
[0067] ⑵Grafted spacer arm
[0068] Add the activated cross-linked agarose gel and 1-3g of 1,6-hexanediamine into a solution of 20-40mL acetone, react in a shaker at 30-37°C, 150-200rpm for 5-8 hours, and react After washing with acetone, unreacted 1,6-hexanediamine was removed, and dried to obtain a cross-linked agarose gel with 1,6-hexamethylenediamine grafted at the end as a spacer arm;
[0069] ⑶Coupling functional ...
Embodiment 3
[0071] Embodiment 3 utilizes 1,2-ethylenediamine as spacer arm coupling ligand
[0072] ⑴Activation
[0073] Take cross-linked agarose gel (GE Healthcare, Sepharose TM CL-6B) 10g, rinse with deionized water several times to remove 20% ethanol, then wash with appropriate amount of acetone for 3 to 5 times, drain and add to 20 In ~40mL acetone solution, activate in a shaker at 25-37°C, 150-200rpm for 2-5 hours, wash with acetone after activation to remove unreacted N,N'-carbonyldiimidazole, and obtain activated Cross-linked agarose gel;
[0074] ⑵Grafted spacer arm
[0075] Add the activated cross-linked agarose gel and 1-3g of 1,2-ethylenediamine into 20-40mL acetone solution, react in a shaker at 150-200rpm at 30-37°C for 5-8 hours, and use it after the reaction Wash with acetone to remove unreacted 1,2-ethylenediamine, and drain to obtain a cross-linked agarose gel with 1,2-ethylenediamine grafted on the end as a spacer arm;
[0076] ⑶Coupling functional ligand
[0077...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com