Synthesis method of colchicine
A technology for colchicine and synthetic methods, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of modification and improvement, low yield of colchicine, etc., achieve simple operation, improve biological activity and Toxic and side effects, the effect of being easy to industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
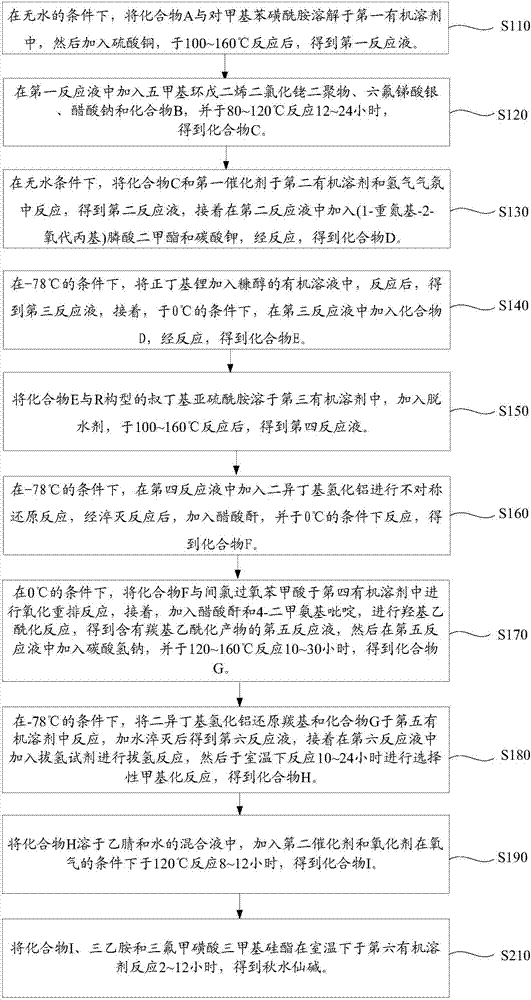
[0029] Such as figure 1 Shown, the synthetic method of the colchicine of one embodiment, comprises the following steps (wherein, Me represented herein is a methyl group, and what NHAc represents is NHCOCH 3 ):
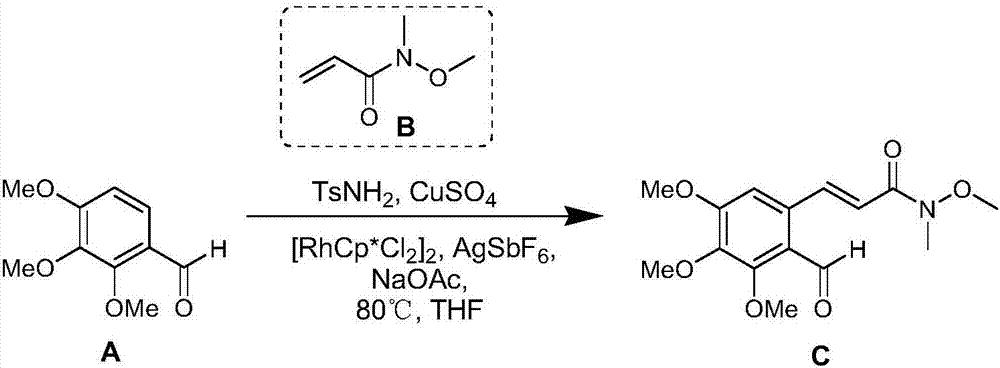
[0030] Step S110: under anhydrous conditions, the structural formula is The compound A and p-toluenesulfonamide are dissolved in the first organic solvent, and then copper sulfate is added to react at 100-160° C. to obtain the first reaction solution.
[0031] Wherein, the molar ratio of compound A to p-toluenesulfonamide is 1:1˜1:1.5.
[0032] Wherein, the first organic solvent is tetrahydrofuran or diethyl ether; preferably tetrahydrofuran, using tetrahydrofuran as the first organic solvent has a higher yield.
[0033] Wherein, copper sulfate is an oxidizing agent and a dehydrating agent in step S110, therefore, using copper sulfate in step S110 can effectively reduce preparation steps and improve efficiency. Specifically, the copper sulfate added in step S110 i...
Embodiment 1
[0079] The preparation process of the colchicine of the present embodiment is as follows:
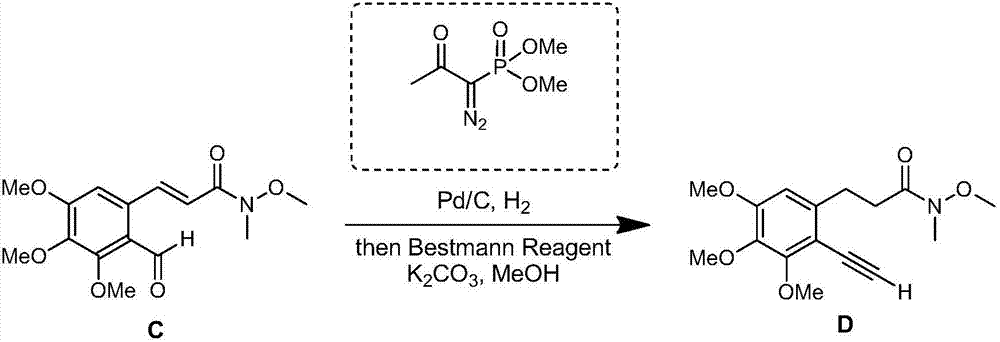
[0080] (1) Dry anhydrous copper sulfate at 100°C for 8 hours to obtain dry anhydrous copper sulfate; then, under nitrogen conditions, dissolve compound A (25.5mmol) and p-toluenesulfonamide (30.6mmol) in To the tetrahydrofuran (100 mL) solution, dry anhydrous copper sulfate (51.0 mmol) was then added, and reacted at 120° C. for 24 hours, and cooled to room temperature to obtain a first reaction solution. In the first reaction solution, add pentamethylcyclopentadiene rhodium dichloride dimer (0.26mmol), silver hexafluoroantimonate (1.04mmol), sodium acetate (51mmol) and compound B (51.0mmol), And reacted at 80°C for 24 hours, the obtained reaction solution was filtered with diatomaceous earth, the filtrate was distilled under reduced pressure to remove tetrahydrofuran, the crude product was dissolved in ethyl acetate, and then washed with water, saturated sodium chloride, anhydrous sodiu...
Embodiment 2
[0105] The preparation process of the colchicine of the present embodiment is as follows:
[0106] (1) Dry anhydrous copper sulfate at 100°C for 12 hours to obtain dry anhydrous copper sulfate; then, under nitrogen conditions, dissolve compound A (25.5mmol) and p-toluenesulfonamide (25.5mmol) in Then add dry anhydrous copper sulfate (127.5 mmol) to the ether (100 mL) solution, react at 100° C. for 24 hours, and cool to room temperature to obtain the first reaction solution. In the first reaction solution, add pentamethylcyclopentadiene rhodium dichloride dimer (0.255mmol), silver hexafluoroantimonate (1.02mmol), sodium acetate (12.75mmol) and compound B (25.5mmol) , and reacted at 120°C for 12 hours, the obtained reaction solution was filtered with diatomaceous earth, the filtrate was distilled under reduced pressure to remove tetrahydrofuran, the crude product was dissolved in ethyl acetate, and then washed with water, saturated sodium chloride in sequence, anhydrous sulfuric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com