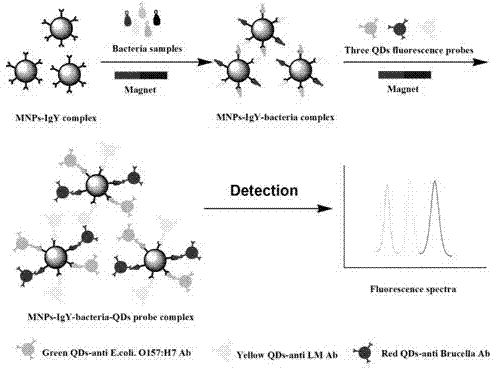
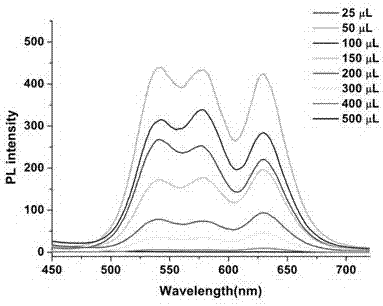
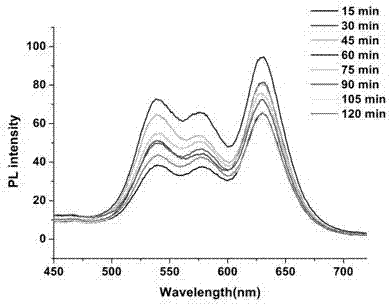
Kit for synchronously and rapidly detecting various zoonotic pathogens
A technology for detecting kits and pathogenic bacteria, which is applied in the direction of measuring devices, instruments, scientific instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0030] Example 1 Preparation of Trivalent Egg Yolk Antibody (IgY)
[0031] Take Escherichia coli O157:H7, Listeria monocytogenes and Brucella strains stored at -80°C, streak them on LA plates after activation, separate individual colonies, and pick individual colonies after culturing at 37°C for 18-24 hours. Inoculate in LB liquid medium and culture with shaking at 37°C until mid-logarithmic phase. Take 1 mL of the bacterial solution and use the plate pouring method to count viable bacteria, add formaldehyde solution with a final concentration of 1% to inactivate at room temperature for 30 min, and overnight at 4 °C. The next day, centrifuge the inactivated bacterial solution at 3000 rpm for 10 min, discard the supernatant, collect the bacterial cells, resuspend the bacterial cells with normal saline to make a bacterial suspension, and adjust the final concentrations of the three bacteria to 2×10 10 CFU mL -1 and stored in a 4°C refrigerator for later use. At the same time,...
Embodiment 2
[0037] Example 2 Preparation of Rabbit Polyclonal Antibody
[0038] Preparation of three kinds of zoonotic pathogenic bacteria rabbit polyclonal antibodies: respectively get Escherichia coli O157:H7 prepared in Example 1, Listeria monocytogenes and Brucella bacterial suspension, add an equal volume of Freund's complete adjuvant Adjuvant / Freund's incomplete adjuvant, put it on a magnetic stirrer and stir until it is completely emulsified, that is, the water-in-oil state. After standing at 4°C for a week, the emulsion does not separate, and it can be used as a vaccine to immunize healthy female New Zealand white rabbits , after a week of adaptive feeding, there was no abnormality, and the back subcutaneous multi-point injection method was used for immunization. Freund's complete adjuvant vaccine was used for the initial immunization, and then the same dose of Freund's incomplete adjuvant vaccine was used for booster immunization once every other week. There were 4 booster immuni...
Embodiment 3 3
[0045] Example 3 Trivalent egg yolk antibody (IgY) indirect ELISA method
[0046] In order to more accurately measure the titer of collected IgY, an indirect ELISA reaction method for detecting trivalent serum and egg yolk antibodies against three different pathogenic bacteria was established. The specific steps are:
[0047] When using Escherichia coli O157:H7 as the coated antigen, dilute the suspension of Escherichia coli O157:H7 bacteria 1:800 with the antigen coating solution, coat 100 μL per well in a 96-well enzyme-labeled reaction plate, and incubate at 37°C 1 h, discard the liquid in the plate, add PBST buffer, wash 300 μL / well, 3 min each time, wash 3 times; add 300 μL 5 % skimmed milk powder to each well, incubate at 37 ° C for 2 h, repeat the above washing steps; To measure trivalent serum or antibody, 100 μL / well, incubate at 37°C for 0.5 h, repeat the above washing steps; dilute the HRP-labeled goat anti-chicken secondary antibody at 1:5000, and add 100 μL / well i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com