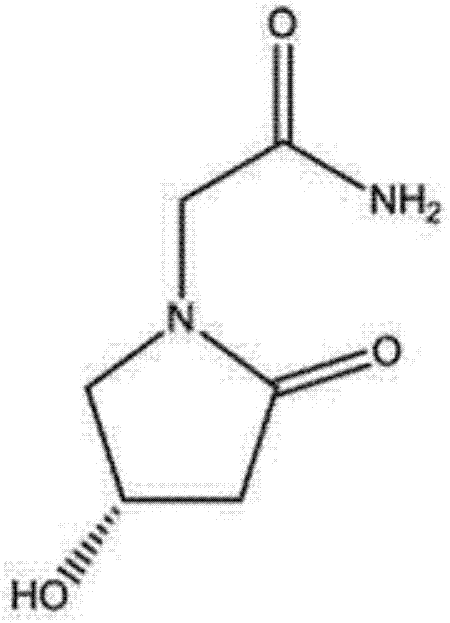
Preparation method for (S)-4-hydroxy-2-oxo-1-pyrrolidine acetamide injection
A pyrrolidine acetamide and injection technology, which is applied in the fields of pharmaceutical formulations, nervous system diseases, drug combinations, etc., can solve the problems of unqualified foreign matter in products, poor product clarity, and reduced product yield, so as to reduce the pain of injection , Patient compliance is good, the effect of improving clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
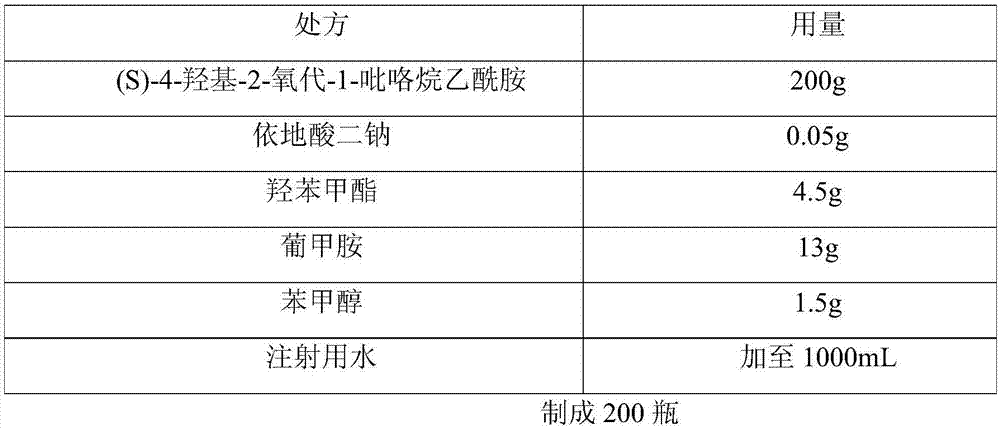
[0024] The prescription of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide injection of embodiment 1 is shown in the table below:
[0025]
[0026] The preparation method of the (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide injection of embodiment 1 comprises the following steps:
[0027] (1) Weigh (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, edetate disodium, methylparaben, meglumine and benzyl alcohol into the water for injection, Stir and dissolve to obtain concentrated liquid; the process of concentrated liquid needs to be treated with nitrogen, and the nitrogen flow rate is 0.03-0.08L / min;
[0028] (2) Take the concentrated solution obtained in step (1), add 0.1mol / L sodium hydroxide solution to adjust the pH value to 6.5;
[0029] (3) add the chitosan of 2g / L in the solution that step (2) obtains, stir and mix, after leaving standstill 50min, filter with the filter membrane of 0.8 μm;
[0030] (4) In the solution that step (3) obtains, add the gac of 1g / L, filter with the...
Embodiment 2
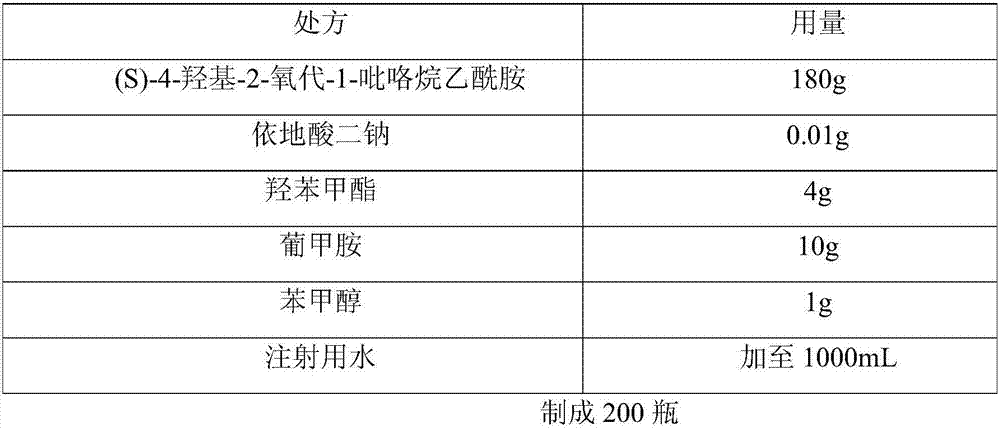
[0039] The prescription of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide injection of embodiment 2 is shown in the table below:
[0040]
[0041] The preparation method of the (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide injection of embodiment 2 comprises the following steps:
[0042] (1) Weigh (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, edetate disodium, methylparaben, meglumine and benzyl alcohol into the water for injection, Stir and dissolve to obtain concentrated liquid; the process of concentrated liquid needs to be treated with nitrogen, and the nitrogen flow rate is 0.03-0.08L / min;
[0043] (2) Take the concentrated solution obtained in step (1), add 0.3mol / L sodium hydroxide solution to adjust the pH value to 6.8;
[0044] (3) add the chitosan of 4g / L in the solution that step (2) obtains, stir and mix, after leaving standstill 40min, filter with the filter membrane of 0.8 μm;
[0045] (4) in the solution that step (3) obtains, add the gac of 2g / L, filter with the...
Embodiment 3
[0054] The prescription of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide injection of embodiment 3 is shown in the following table:
[0055]
[0056] The preparation method of the (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide injection of embodiment 3 comprises the following steps:
[0057] (1) Weigh (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, edetate disodium, methylparaben, meglumine and benzyl alcohol into the water for injection, Stir and dissolve to obtain concentrated liquid; the process of concentrated liquid needs to be treated with nitrogen, and the nitrogen flow rate is 0.03-0.08L / min;
[0058] (2) Take the concentrated solution obtained in step (1), add 0.5mol / L sodium hydroxide solution to adjust the pH value to 7.0;
[0059] (3) add the chitosan of 6g / L in the solution that step (2) obtains, stir and mix, after standing 30min, filter with the filter membrane of 0.8 μ m;
[0060] (4) Add the gac of 3g / L in the solution that step (3) obtains, filter with the filte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com