Application of Cyclodextrin in the Preparation of Radioactive Iodine Labeled Hypericum Drug Preparations
The technology of a pharmaceutical preparation and radioactive iodine is applied in the application field of cyclodextrin in the preparation of radioactive iodine-labeled hypericum drug preparations, and can solve the problems of not obtaining and the like, and achieve low cost, high preparation efficiency and fast blood removal. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Traditional formulations (DMSO- 131 I-Hyp is diluted with equal proportions of PEG and propylene glycol) Drug preparation:
[0031] First, weigh 0.01-13mg of Hyp (1 mg is the best), and add 1 mL of DMSO in proportion to obtain 0.01-13 mg / mL DMSO-Hyp solution drug (1 mg / mL is the best). Apply the catalyst Iodogen to the 1.5mL EP tube in advance (the best ratio is 3-10ug Iodogen:1mCi Na 131 1), add about 1mL of DMSO-Hyp solution, then add about 1-100μL of Na 131 I solution (the specific activity is 20-1000mCi / mL, the half-life of the isotope from production to use is not more than 1, and the best treatment is 100μL), vortex and mix continuously and check the labeling rate until the labeling rate reaches more than 98%. Dilute with 1mL PEG and 1mL propylene glycol to obtain DMSO- 131 PEG, Propylene Glycol Diluent of I-Hyp.
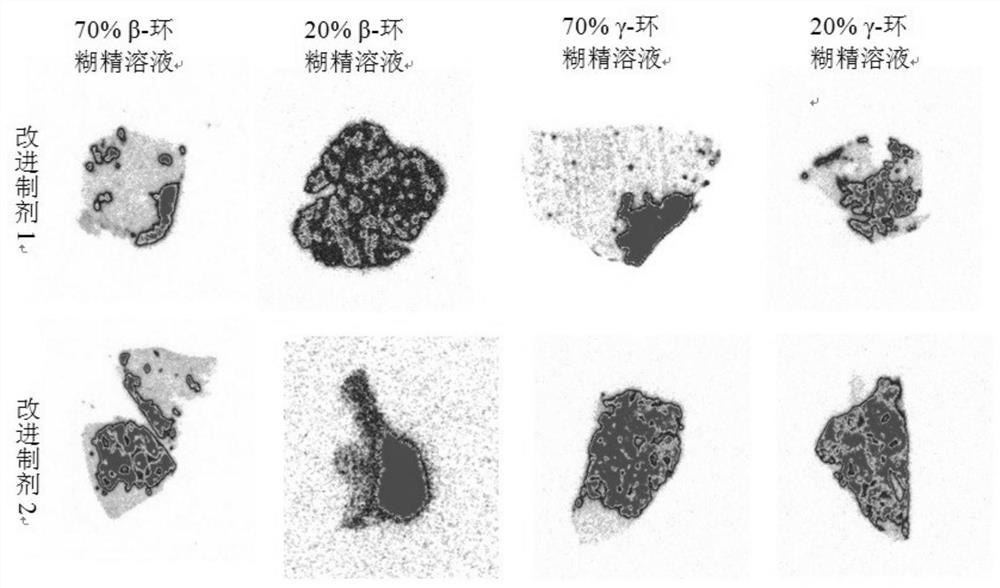
[0032] Improved formulation 1 (cyclodextrin- 131 I-Hyp solution) drug preparation:
[0033] First weigh 0.01-1mg of Hyp, add 1mL of 50% mass volu...
Embodiment 2
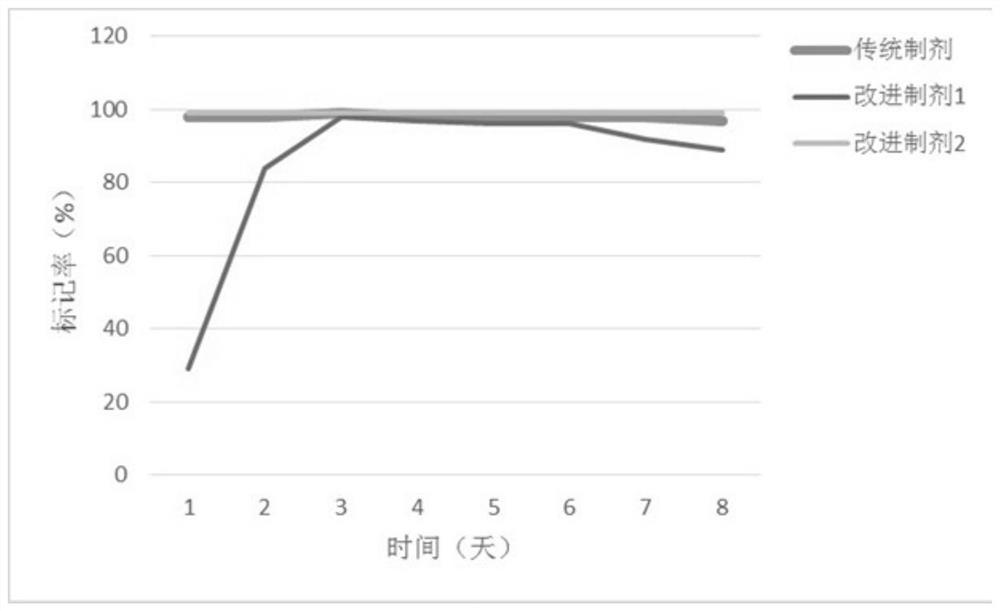
[0037] Comparison experiment of labeling rate between traditional preparation and improved preparation
[0038] The traditional preparation, improved preparation 1, and improved preparation 2 described in Example 1 were compared for labeling rate within 7 days; Solution to cyclodextrin-saturated Hyp solution, 0.01-10mg / mL DMSO-Hyp solution by adding Na 131 I solution, after mixing for a period of time under the catalyst lodogen, adopt thin-layer chromatography (Thin-layer chromatography, TLC), coordinate gamma counter to detect the concentration of solution 131 I mark rate.
[0039] Then add 0.01-13mg / mL DMSO- 131 I-Hyp solution was diluted with equal volume of PEG and propylene glycol to obtain traditional preparations; 0.01-10mg / mL DMSO- 131 I-Hyp is diluted with at least 15 times of 50% mass volume fraction of hydroxypropyl-β-cyclodextrin solution to obtain improved formulation 2, and with improved formulation 1 (cyclodextrin- 131 I-Hyp solution) for stability testing o...
Embodiment 3
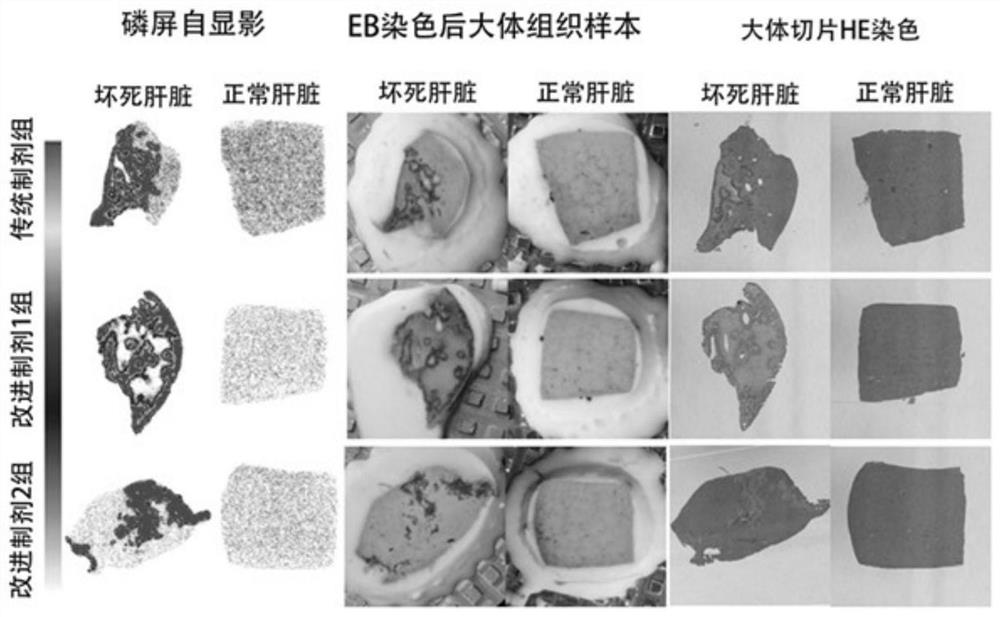
[0042] Traditional preparation and improved preparation 1, improved preparation 2 used in rat experiments 131 I-Hyp biodistribution and necrosis targeting comparison experiment
[0043] Model building:
[0044] Male SD rats (250-300 g) began to take 0.5% KI solution instead of drinking water 24 hours before operation to block the thyroid uptake of free iodine. On the day of the operation, a laparotomy was performed under anesthesia for necrosis and reperfusion of the right liver lobe. Within 24 hours, an MRI scan was performed to detect whether there was local necrosis of the liver.
[0045] Selected 18 hepatic necrosis-reperfusion model rats and randomly divided them into 3 groups, 6 rats per group.
[0046] Prepare traditional preparation, improved preparation 1, and improved preparation 2 according to the best (optimum) concentration of embodiment 1, and inject about various preparations (Hyp dosage is 0.1mg / Kg) about 100 μ Ci respectively in penis vein, before putting to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com