Application of cyclodextrin in preparing radioactive iodine labeling hypericum monogynum medicine preparation
A pharmaceutical preparation and radioactive iodine technology are applied in the application field of cyclodextrin in the preparation of radioactive iodine-labeled hypericum drug preparations, can solve the problems of not obtaining and the like, and achieve the advantages of low cost, guaranteed labeling efficiency and fast blood removal. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Traditional formulations (DMSO- 131 I-Hyp is diluted with equal proportions of PEG and propylene glycol) drug preparation:
[0031] First weigh 0.01-13mg Hyp (optimally 1mg), add 1mL DMSO in proportion to obtain 0.01-13mg / mL DMSO-Hyp solution drug (optimally 1mg / mL). Apply the catalyst Iodogen to a 1.5mL EP tube in advance (the optimal ratio is 3-10ug Iodogen:1mCi Na 131 1), add about 1 mL of DMSO-Hyp solution, then add about 1-100 μL of Na 131 I solution (specific activity is 20-1000mCi / mL, the isotope does not exceed 1 half-life from production to use, and the best is 100μL during treatment), vortex and mix continuously and detect the labeling rate until the labeling rate reaches more than 98%, and then Dilute with 1mL PEG and 1mL propylene glycol to obtain DMSO- 131 PEG, propylene glycol diluent of I-Hyp.
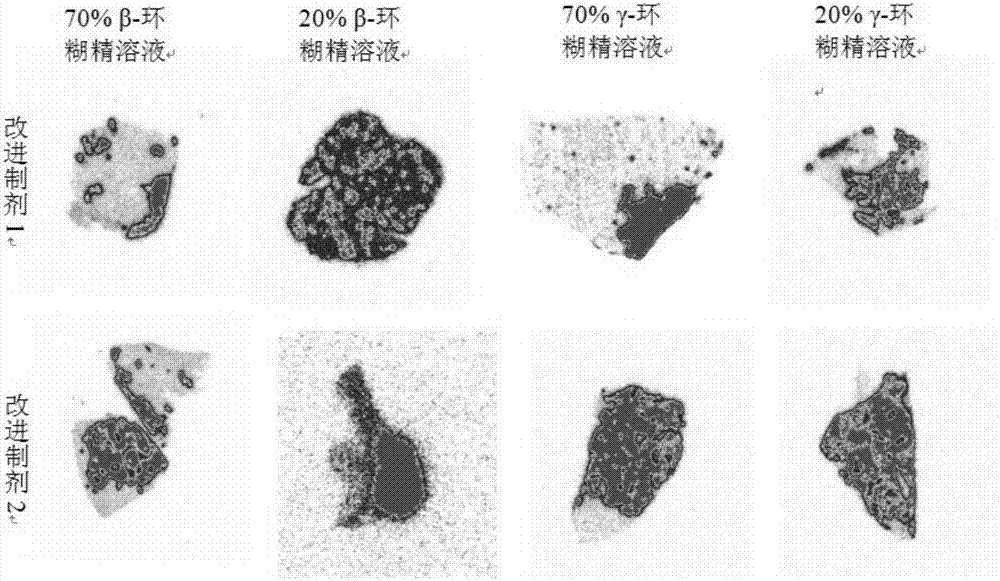
[0032] Improved formulation 1 (cyclodextrin- 131 I-Hyp solution) drug preparation:
[0033] First, weigh 0.01-1mg Hyp, add 1mL of 50% hydroxypropyl-β-cyclod...
Embodiment 2
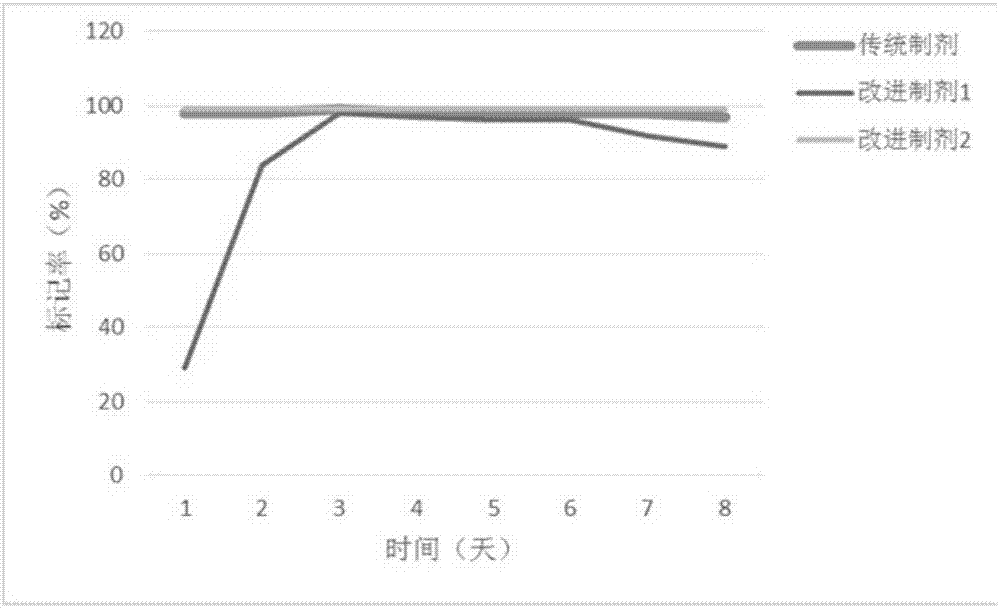
[0037] Contrastive experiment of labeling rate of traditional preparation and improved preparation
[0038] The traditional formulation, improved formulation 1, and improved formulation 2 described in Example 1 were compared for the labeling rate within 7 days; the specific operations were: 0.01-13 mg / mL DMSO-Hyp solution, 0.01 mg / mL cyclodextrin-Hyp solution solution to cyclodextrin-saturated Hyp solution, 0.01-10 mg / mL DMSO-Hyp solution was added with Na 131 I solution, after mixing for a period of time under catalyst Iodogen, adopt thin-layer chromatography (Thin-layer chromatography, TLC), cooperate with gamma counter to detect the concentration of solution 131 I labelling rate.
[0039] Then 0.01-13 mg / mL DMSO- 131 I-Hyp solution is diluted with equal volume of PEG and propylene glycol to obtain traditional preparation; 0.01-10mg / mL DMSO- 131 I-Hyp is diluted with at least 15 times the 50% mass and volume fraction of hydroxypropyl-β-cyclodextrin solution to obtain impr...
Embodiment 3
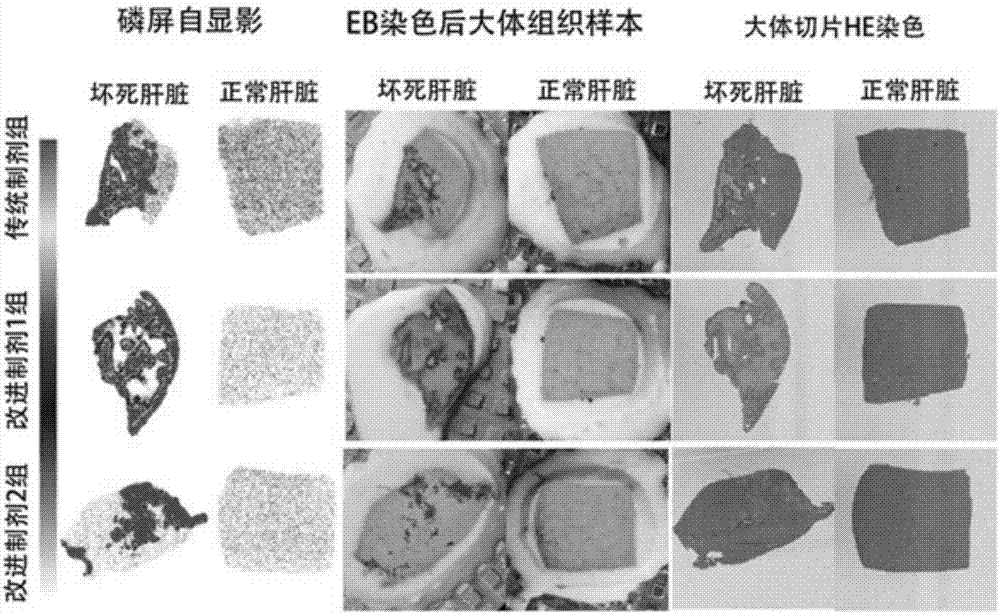
[0042] Traditional formulation and improved formulation 1 and improved formulation 2 were used in rat experiments 131 I-Hyp biodistribution and necrosis targeting comparative experiment
[0043] Model building:
[0044] Male SD rats (250-300 g) were given 0.5% KI solution instead of drinking water 24 hours before surgery to block the thyroid uptake of free iodine. On the day of the operation, anesthesia and laparotomy were performed, and a right hepatic lobe necrosis and reperfusion operation was performed. Magnetic resonance scanning was performed within 24 hours to detect whether the local liver was necrotic.
[0045] Eighteen hepatic necrosis-reperfusion model rats were selected and randomly divided into 3 groups, 6 rats / group.
[0046] According to the best (optimal) concentration of Example 1, the traditional preparation, the improved preparation 1, and the improved preparation 2 were prepared, and about 100 μCi of various preparations (Hyp dose of 0.1 mg / Kg) were injec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com