Sex-attractant halogenated analogue for preventing agrotis ypsilon
A technology of small cutworms and analogues, applied in the fields of attracting pests, chemicals for biological control, pest control, etc., achieving the effects of simple preparation, good stability, and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
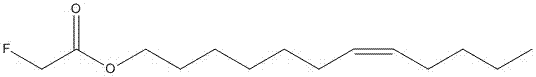
[0026] Embodiment 1 The preparation of cis-7-dodecenyl-2-monofluoroacetate
[0027] The compound in the present invention can be synthesized by the route described below, the synthetic route of cis-7-dodecenyl-2-monofluoroacetate see Figure 4 .
[0028] Dissolve 0.5g (0.002mol) of cis-7-dodecenol in 20mL of anhydrous dichloromethane, add 0.04g of 4-dimethylaminopyridine, then add 0.31g of TEA, ice-bath, and react The temperature is maintained at about 0°C, and the dichloromethane solution of fluoroacetic anhydride is gradually added dropwise. After the dropwise addition, the ice bath lasts for a period of time, and the plate is spotted to determine whether the reaction is complete. After the reaction was complete, impurities and excess acid anhydride were removed by extraction with saturated brine and anhydrous sodium sulfate. Finally, the solvent was removed by rotary evaporation under reduced pressure, and 0.61 g (1.87 mmol) of light yellow oil was obtained by column chrom...
Embodiment 2
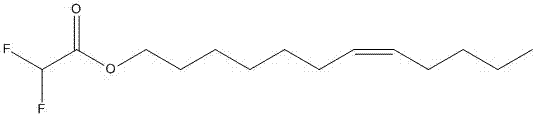
[0029] Example 2 Preparation of cis-7-dodecenyl-2,2-difluoroacetate
[0030] The preparation method of cis-7-dodecenyl-2,2-difluoroacetate is similar to Example 1, only need to change the chloroacetic anhydride in Example 1 into difluoroacetic anhydride, and according to Example Synthesize according to the steps in 1 to obtain 0.57 g (1.82 mmol) of light yellow oil with a yield of 78.9%.
Embodiment 3
[0031] Embodiment 3 field bioactivity assay test
[0032] Lure preparation:
[0033] (1) The lure carrier adopts a cylindrical green concave rubber plug with a height of 14mm, an inner diameter of the opening end of 8mm, a wall thickness of the opening end of 1mm, and an opening depth of 5mm.
[0034] (2) The sex pheromone components of the cutworm were cis-7-dodecyl acetate (Z7-12:Ac), cis-9-tetradecyl acetate (Z9-14:Ac), cis- 11-Hexadecyl acetate (Z11-16:Ac) was mixed at a mass ratio of 3:1.3:0.8, and diluted with n-hexane to a solution with a concentration of 1 μg / μL.
[0035] (3) Pipette 100 μL (1 μg / μL) of the above-mentioned cutworm sex pheromone solution and add it to the bottom of the rubber head, and the little cutworm sex pheromone solution will penetrate into the rubber with the solvent (n-hexane). After the solvent (n-hexane) was volatilized, 100 μg of the sex pheromone of the cutworm was obtained.
[0036] (4) cis-7-dodecenyl-2-monofluoroacetate, cis-9-tetradec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com