Diazepam-D5 preparation method
A technology of diazepam and compounds, which is applied in the field of preparation of deuterated diazepam standard intermediates, can solve the problems of high price, limited wide use, poor stability of deuterated internal standards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
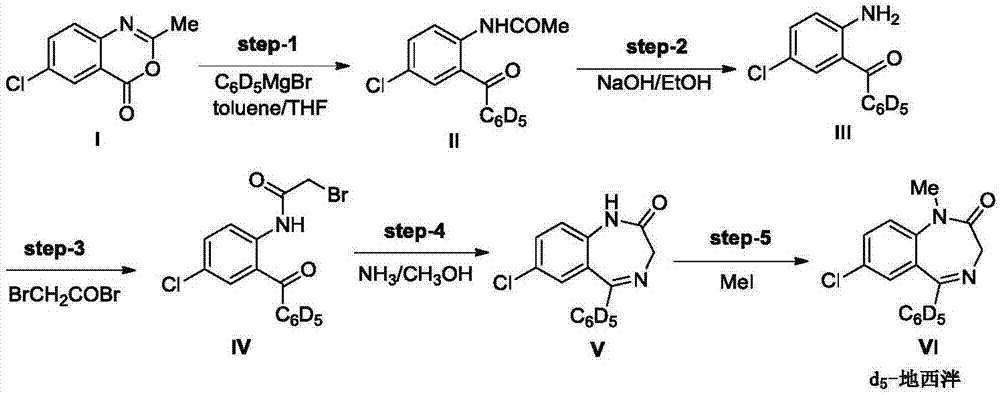
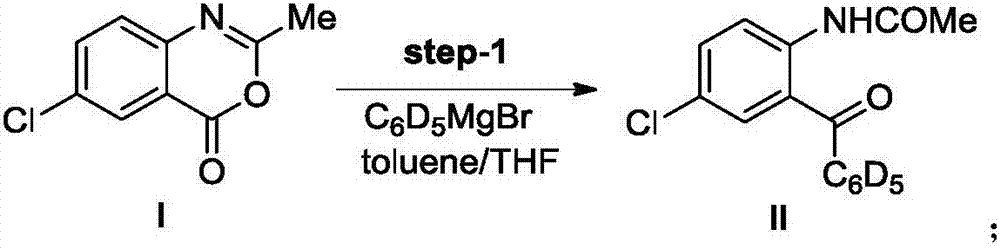
[0033] Step (1): 2-acetamide-5-chlorobenzophenone-2',3',4',5',6'-d 5 (Formula II compound) preparation
[0034]
[0035] Put 2.6g of Mg strips in a 250mL three-necked flask, add 50mL of anhydrous THF, then add 30mg of iodine particles, add a THF solution of deuterated bromobenzene dropwise under stirring (8.8g of deuterated bromobenzene is added to 40mL THF), and reflux the reaction After 1 hour, the Grignard reagent was prepared.
[0036] Put 10.0g of raw material 6-chloro-2-methyl-4H-3,1-benzoxazin-4-one (compound of formula I) in a 250mL single-necked bottle, then add 50mL of toluene, and cool down to below 0°C in an ice bath , Add the above-mentioned Grignard reagent dropwise, and the dropping time is greater than 45min. After the addition is complete, stir at 0°C for 30 minutes, then rise to room temperature and stir overnight, then cool the reaction to 0°C, add 100 mL of 6mol / L dilute hydrochloric acid, stir for 30 minutes and then separate the liquids, extract the ...
Embodiment 2
[0052] Embodiment 2 (preparation method of formula V compound)
[0053] Steps (1), (2) are the same as in Example 1.
[0054] Step (3): 2-bromoacetamide-5-chlorobenzophenone-2',3',4',5',6'-d 5 (Formula IV compound) preparation:
[0055]
[0056] Put 7.08g of the compound of formula III in a 250mL round-bottomed flask, add 36mL of toluene to dissolve it, then add 4.74g of pyridine, cool down to 0°C in an ice bath, add dropwise a toluene solution of bromoacetyl bromide (3.9 mL of bromoacetyl bromide is added to 20 mL of chloroform Middle), after TLC monitors that the reaction is completed, add water to quench the reaction, extract the organic phase with toluene (30 mL each time, 3 times in total), combine the organic phases and wash with saturated sodium carbonate, dry and concentrate under reduced pressure to obtain an oily substance, which is The crude product of the compound of formula IV was directly used in the next reaction without purification.
[0057] Step (4): 7-...
Embodiment 3
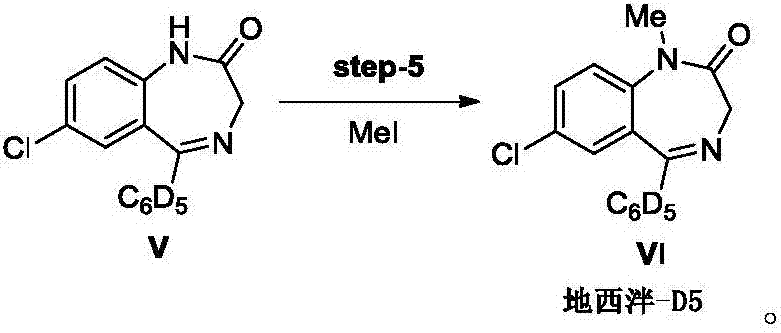
[0062] The difference between this example and Example 1 lies in step (5): diazepam-D5: 7-chloro-1,3-dihydro-1-methyl-5-(phenyl-d 5 )-2H-1, the preparation of 4-benzodiazepine-2-ketone (formula VI compound):
[0063]
[0064] The compound of formula V (5.5g) was dissolved in DMF (30mL), cooled to -5°C in an ice-salt bath, NaH (0.48g) was added and stirred for 15min. At this time, the system was light yellow and transparent. The DMF solution of methyl iodide (2.8g methyl iodide is dissolved in 25mL DMF) is slowly added dropwise to the reaction system (methyl iodide is dissolved in DMF and slowly added dropwise, in order to avoid producing the by-products of the two methyl groups, separation Purification is difficult and the yield is low), the dropwise addition is completed, and the reaction takes 30 minutes. Then slowly add saturated ammonium chloride to quench the reaction, after extraction with ethyl acetate, combine the organic phases, wash with water three times, after ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com