Preparation method of 2,2-difluoro-1,3-propanediol
A technology of difluoropropanol and propylene glycol, which is applied in the field of preparation of 2,2-difluoro-1,3-propanediol, can solve the problems of high synthesis cost, limited practical application, and difficulty in obtaining raw materials, and achieves good stability, Simple equipment requirements and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
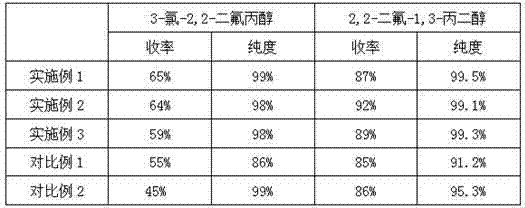
Embodiment 1
[0024] 1.1 Preparation of 2-chloro-1,1-difluoroethylene
[0025] Add 1200g of 30% sodium hydroxide aqueous solution and 2.5g of tetrabutylammonium bromide into a 2000ml three-necked flask, and add 500g of 1,2-dichloro-1,1-difluoroethane while stirring with a dropping funnel, React at 30°C for 6h, then pass the gas through the condenser tube into the collection bottle of carbon tetrachloride, and the collection bottle is cooled to minus 20°C by dry ice. The weight of the collection bottle increased by about 300g; the purity of the reaction gas 2-chloro-1,1-difluoroethylene detected by gas chromatography was 98%;
[0026] 1.2 Preparation of 3-chloro-2,2-difluoropropanol
[0027] The 3L reaction kettle was condensed and cooled by the coil, vacuumized, and 500g of carbon tetrachloride solution of 2-chloro-1,1-difluoroethylene with a mass concentration of 22.6% in the cold well was sucked into the kettle, which contained 2-chloro- Add 113g of 1,1-difluoroethylene, add 1101g of me...
Embodiment 2
[0031] 2.1 Preparation of 2-chloro-1,1-difluoroethylene
[0032] In a 2000ml three-necked flask, add 1166g of 30% aqueous sodium hydroxide solution, 5g of tetrabutylammonium bromide, and add 1,2-dichloro-1,1-difluoroethane 500g while stirring with a dropping funnel, in React at 30°C for 6 hours, then pass the gas through the condenser tube into the collection bottle with 1 kg of carbon tetrachloride remaining, and cool the collection bottle to minus 25°C with dry ice. The weight of the collection bottle increased by about 300g; the purity of the reaction gas 2-chloro-1,1-difluoroethylene detected by gas chromatography was 99%;
[0033] 2.2 Preparation of 3-chloro-2,2-difluoropropanol
[0034] The 3L reaction kettle was condensed and cooled by the coil, vacuumized, and 500g of carbon tetrachloride solution of 2-chloro-1,1-difluoroethylene with a mass concentration of 22.6% in the cold well was sucked into the kettle, which contained 2-chloro- Add 113g of 1,1-difluoroethylene,...
Embodiment 3
[0038] 3.1 Preparation of 2-chloro-1,1-difluoroethylene
[0039] Add 1200g of 30% sodium hydroxide aqueous solution and 3.8g of tetrabutylammonium bromide into a 2000ml three-necked flask, and add 500g of 1,2-dichloro-1,1-difluoroethane while stirring with a dropping funnel, React at 30°C for 6h, then pass the gas through the condenser tube into the collecting bottle with 1kg of carbon tetrachloride remaining, and the collecting bottle is cooled to minus 25°C by dry ice. The weight of the collection bottle increased by about 300g; the purity of the reaction gas 2-chloro-1,1-difluoroethylene detected by gas chromatography was 98%;
[0040] 3.2 Preparation of 3-chloro-2,2-difluoropropanol
[0041] The 3L reaction kettle was condensed and cooled by the coil, vacuumized, and 500g of carbon tetrachloride solution of 2-chloro-1,1-difluoroethylene with a mass concentration of 22.6% in the cold well was sucked into the kettle, which contained 2-chloro- Add 113g of 1,1-difluoroethyle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com