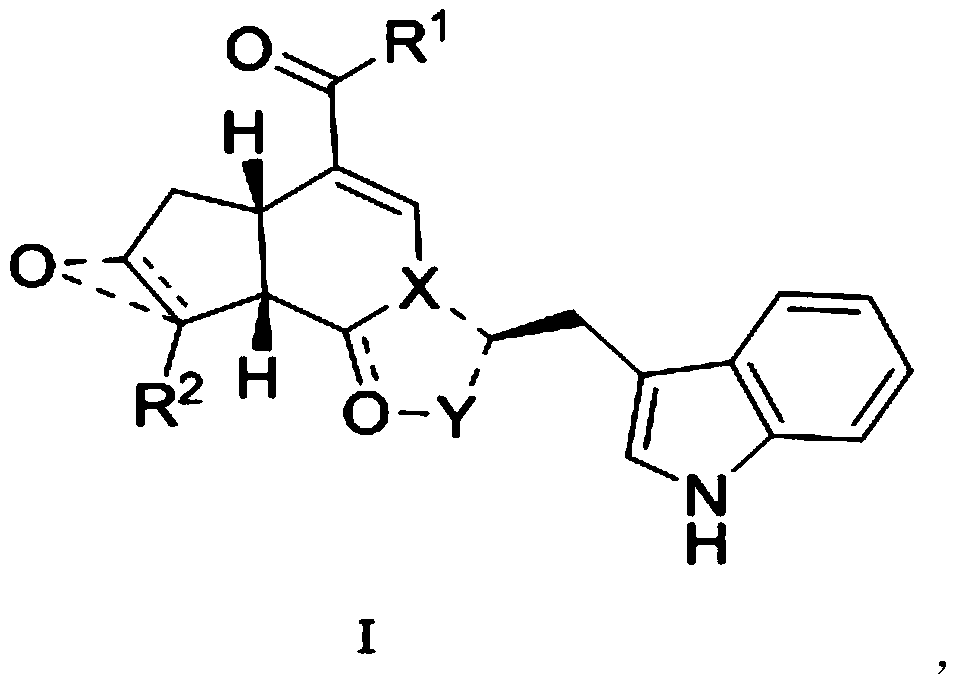
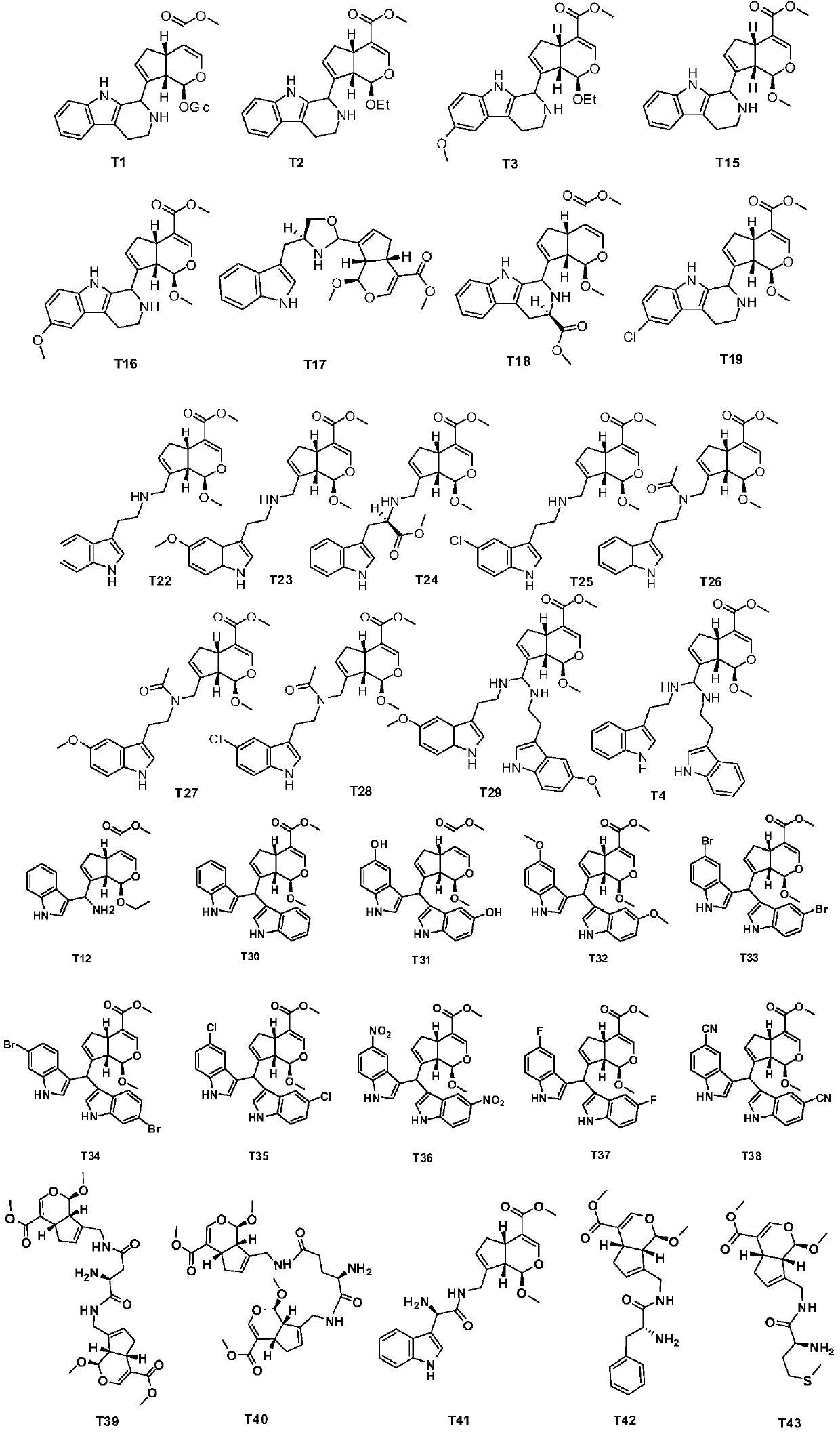
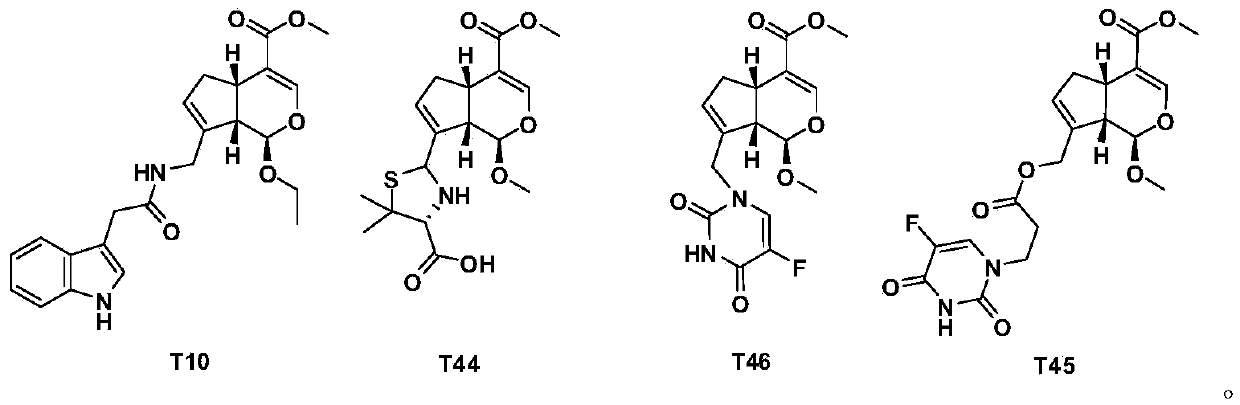
Pseudomonoterpene indole alkaloid and its preparation method and application
A kind of pseudo-monoterpene indole biology, the technology of monoterpene indole biology, applied in the field of pseudo-monoterpene indole alkaloid and its preparation, can solve the problem of high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The preparation of embodiment 1 compound T1 comprises the following steps:
[0061] Step (1): Take 1.77mmol geniposide (geniposide) and put it into a 100mL dry round bottom flask, add 50mL dry tetrahydrofuran, then add activated manganese dioxide in batches, move to a 50°C oil bath and stir, the second Day treatment: filter (diatomaceous earth) to remove manganese dioxide and evaporate the solvent under reduced pressure, and the residue is separated by column chromatography (chloroform:methanol=10:1) to obtain the product M8.
[0062] Step (2): In the 100mL round bottom flask that 0.29mmol M8 is housed, add the H of 0.29mmol tryptamine hydrochloride and 0.14mmol tryptamine (Tryptamine) 2 O / AcOH (15mL / 0.73mL) solution, under nitrogen protection, was placed in an oil bath at 100°C and stirred overnight. The solvent was evaporated to dryness under reduced pressure, and the residue was separated by column chromatography (chloroform-methanol-diethylamine=200:20:1) and prepa...
Embodiment 2
[0064] The preparation of embodiment 2 compound T2 comprises the following steps:
[0065] Step (1): Weigh 0.88mmol of Genipin (Genipin), put it into a 25mL round bottom flask, add 4mL of absolute ethanol, then add a drop of concentrated hydrochloric acid, and stir at 60°C for 6h. 1 mol / L sodium hydroxide was added to the reaction solution to adjust the pH to 7, concentrated under reduced pressure, the residue was extracted with ethyl acetate, the extract was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was separated by silica gel column chromatography (petroleum ether-ethyl acetate=1:1) to obtain product M9.
[0066] Step (2): Take 3.93mmol M9 into a 250mL round bottom flask, add 30mL dry dichloromethane, add 2.27mmol Dess Martin oxidant (DMP) after dissolving, stir at room temperature for 1h, add saturated sodium bicarbonate, saturated thio Sodium sulfate aqueous solution, stir well. Th...
Embodiment 3
[0069] The preparation of embodiment 3 compound T3, comprises the following steps:
[0070] In a 10mL round bottom flask containing 0.13mmol 5-methoxytryptamine (M10A), add 0.13mmol 5-methoxytryptamine, 10mL ethanol, stir at room temperature for 1h, add a drop of concentrated hydrochloric acid, stir overnight, add saturated carbonic acid Sodium hydrogen aqueous solution to neutralize the acid, add water, extract with ethyl acetate, wash with water and saturated brine, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and the residue is subjected to preparative thin-layer chromatography (ethyl acetate-methanol-di Ethylamine=100:1:1) to isolate the product T3.
[0071] The yield of compound T3 was 43.5%. ESI-MS m / z: 424.2[M+H] + ; 1 H-NMR (CDCl 3 , 400MHz) δ(ppm): 7.25(s, 1H), 7.50(s, 1H), 7.21(d, J=8.8Hz, 1H), 6.94(d, J=2.4Hz, 1H), 6.83(dd, J=8.8, 2.4Hz, 1H), 5.89(s, 1H), 5.01(s, 1H), 4.78(d, J=8.0Hz, 1H), 4.06-4.02(m, 1H), 3.86(s, 3H ), 3.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com