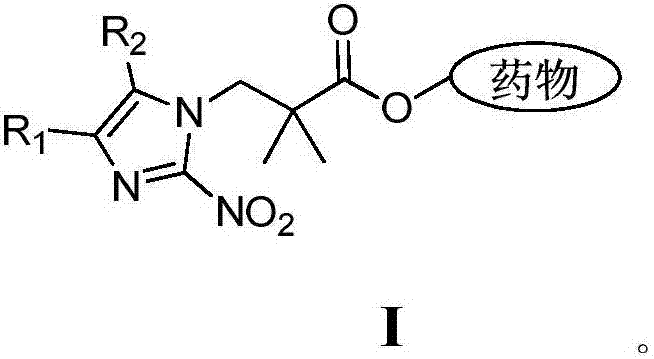
Hypoxic activation prodrug based on 2,2-dimethyl-3-(2-nitroimidazolyl) propionic acid
A nitroimidazolyl and dimethyl technology, applied in the field of hypoxia-activated prodrug of 2,2-dimethyl-3-propionic acid and its synthesis, can solve the problems of unstable structure and low synthesis yield , to achieve the effects of improving targeting, enhancing curative effect, and convenient and efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
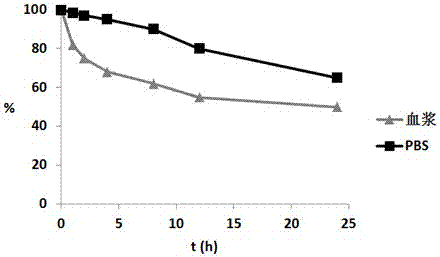
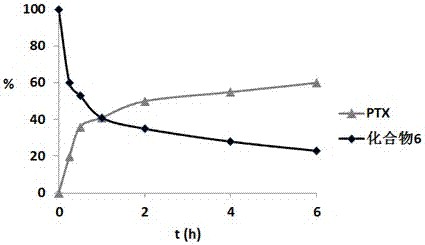
Image
Examples
Embodiment 1
[0038] Preparation of compound 2
[0039]
[0040] Diisopropylamine (15.4 mL, 0.11 mol) was dissolved in anhydrous tetrahydrofuran (100 mL), the mixture was cooled to -30°C, and n-butyllithium (44.0 mL, 0.106 mol) was slowly added dropwise to the system. After the dropwise addition, the reaction system was reacted at 0°C for 30 minutes, cooled to -78°C again, compound 1 (13.6mL, 0.10mol) was added dropwise to the reaction system, and reacted at this temperature for 1 hour and then added to the mixed system Diiodomethane (5.20 mL, 0.065 mol) was added dropwise, and the reaction mixture was reacted at room temperature for 16 hours, and compound 1 was completely reacted. The reaction mixture was poured into saturated aqueous ammonium chloride (100 mL), extracted with ethyl acetate, and the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and concentrated. The concentrate was distilled by an oil pump under reduced pressure to obtain compound 2 (25.0...
Embodiment 2
[0042] Preparation of Compound 4
[0043]
[0044] Cesium carbonate (2.88g, 8.84mmol) was added to a solution of compound 3 (0.500g, 4.42mmol) in N,N-dimethylformamide (15mL), the mixture was stirred at room temperature for 15 minutes, and the compound was added to the system 2 (2.26 g, 8.84 mmol). The reaction mixture was reacted at 100° C. for 16 hours, the color of the reaction solution changed from yellow to brownish red, and the compound 3 was completely reacted. The reaction solution was cooled to room temperature, ethyl acetate (60 mL) was added to the system, filtered, the filtrate was washed with saturated sodium bicarbonate (30 mL), the organic phase was dried with anhydrous sodium sulfate, filtered, concentrated, and the concentrate was passed through column chromatography ( Petroleum ether:ethyl acetate=3:1) Separation and purification gave light yellow oil 4 (1,1 g, 98%). 1 H NMR (400MHz, CDCl 3 )δ7.13(s, 1H), 7.11(s, 1H), 4.74(s, 2H), 4.15(q, J=7.2Hz, 2H), ...
Embodiment 3
[0046] Preparation of compound 5
[0047]
[0048] Lithium hydroxide monohydrate (418 mg, 9.96 mmol) was added in batches to a mixed solution of compound 4 (600 mg, 2.49 mmol) in methanol (20 mL) and water (4 mL), and the reaction system was stirred at room temperature for 16 hours to complete the reaction. The methanol in the system was removed by a water pump, the pH of the mixed system was adjusted to 4.0 with dilute hydrochloric acid (2N) solution, the aqueous phase was extracted with ethyl acetate (30mL*2), the organic phase was washed with saturated aqueous sodium chloride solution, anhydrous sulfuric acid Dry over sodium, filter and concentrate to give 5 (448 mg, 84%) as a pale yellow solid. 1 H NMR (400MHz, CDCl 3)δ7.21(s,1H),7.16(s,1H),4.76(s,2H),1.27(s,6H), MS(ESI)m / z=213.08[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com