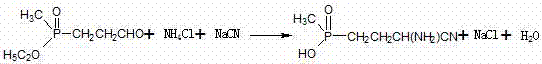Addition process of hydrocyanic acid
A hydrocyanic acid and addition technology, applied in chemical instruments and methods, organic chemistry, compounds of Group 5/15 elements of the periodic table, etc., can solve problems such as difficult handling, and achieve increased reaction yield, improved solubility, The effect of shortening the one-pot reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]A hydrocyanic acid addition process, in the cyanamide reaction kettle, put in 1,2-dichloroethane as a solvent, and then put in sodium cyanide solid, so that the input sodium cyanide solid and 1,2-dichloroethane After ethyl chloride is uniformly mixed at a molar ratio of 0.15:1, the molar ratio of sodium cyanide solid to phosphonic aldehyde is 0.98:1 and then put into the phosphonic aldehyde liquid, so that the sodium cyanide solid and phosphonic aldehyde liquid are at 1, 2 -After dissolving in ethylene dichloride, pass into ammonia gas in the suspension of this solution, control the feeding rate of ammonia gas to be 20kg / h, the feeding time is 2.5h, the solution in the still that makes becomes clear and transparent. Can.
[0036] Control the above reaction time to 4 hours, and the reaction temperature is 30°C. After the reaction is completed, carry out vacuum distillation on the materials after the reaction at -0.06Mpa and 40°C to distill out 1,2-dichloroethane and untre...
Embodiment 2
[0038] A hydrocyanic acid addition process, in the cyanamide reaction kettle, put in 1,2-dichloroethane as a solvent, and then put in sodium cyanide solid, so that the input sodium cyanide solid and 1,2-dichloroethane After ethyl chloride is evenly mixed at a molar ratio of 0.2:1, the molar ratio of sodium cyanide solid to phosphonic aldehyde is 0.98:1, and the phosphonic aldehyde solution is put into it, so that the sodium cyanide solid and phosphonic aldehyde liquid are at 1, 2 - After dissolving in ethylene dichloride, feed ammonia gas into the suspension of the solution, control the feeding rate of ammonia gas to 40kg / h, and the feeding time is 3h, so that the solution in the kettle becomes clear and transparent .
[0039] Control the above reaction time to 6 hours, and the reaction temperature is 40°C. After the reaction is completed, the material after the reaction is subjected to vacuum distillation at -0.08Mpa and 50°C to distill 1,2-dichloroethane and untreated After...
Embodiment 3
[0041] A hydrocyanic acid addition process, in the cyanamide reaction kettle, put in 1,2-dichloroethane as a solvent, and then put in sodium cyanide solid, so that the input sodium cyanide solid and 1,2-dichloroethane After ethyl chloride is uniformly mixed at a molar ratio of 0.19:1, the molar ratio of sodium cyanide solid to phosphonic aldehyde is 0.98:1, and the phosphonic aldehyde solution is put into it, so that the sodium cyanide solid and phosphonic aldehyde liquid are at 1, 2 -After dissolving in ethylene dichloride, pass into ammonia gas in the suspension of this solution, control the feeding rate of ammonia gas to be 30kg / h, and the feeding time is 2.6h, make the solution in the kettle become clear and transparent. Can.
[0042] Control the above reaction time to 5 hours, and the reaction temperature is 35°C. After the reaction is completed, carry out vacuum distillation on the materials after the reaction at -0.07Mpa and 45°C to distill out 1,2-dichloroethane and un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com