Synthesis method of bambuterol hydrochloride impurity D
A technology of bambuterol hydrochloride and its synthesis method, which is applied in the field of organic chemical synthesis, can solve the problems of less impurity D in bambuterol hydrochloride, etc., and achieve the effect of easy-to-obtain raw materials, easy operation, and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
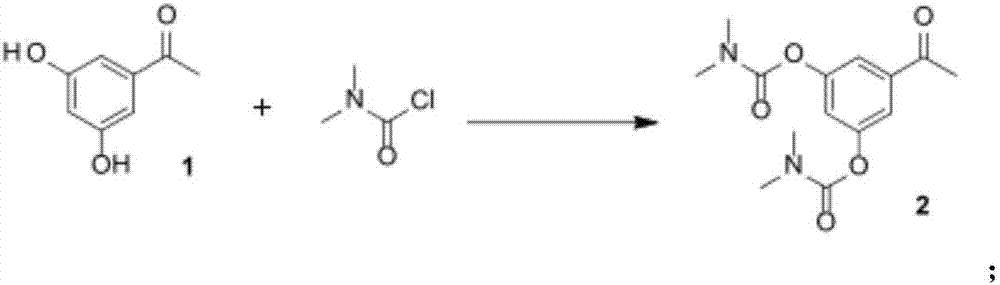
[0044] Dissolve 25 g of the compound of formula 1 [1-(3,5-dihydroxyphenyl)ethanone] in 200 ml of ethyl acetate, add 90 g of potassium carbonate and 10 ml of pyridine. Weigh 47 g of N,N-dimethylcarbamoyl chloride and dissolve it in 60 ml of ethyl acetate, and slowly drop it into the reaction system at room temperature. After the dropwise addition was completed, the mixture was stirred overnight at room temperature. Add 200 ml of purified water and stir well for 1 hour, separate liquid extraction, extract the aqueous phase with ethyl acetate (100 ml*2), combine the organic phases, and wash with 200 ml of 2% H 2 SO 4 Wash once with aqueous solution, once with 200 ml of saturated brine, dry over anhydrous sodium sulfate for 2 hours, filter, concentrate under reduced pressure to remove ethyl acetate, and obtain the compound of formula 2 [5-acetyl-1,3-phenylene- Bis(N,N-dimethylcarbamate)] 42 g, yield: 86.9%, purity: 98%.
[0045] The characterization data of the compound of gain...
Embodiment 2
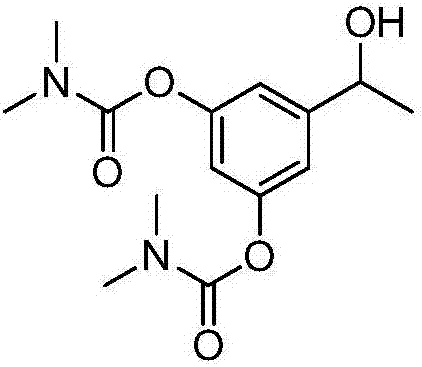
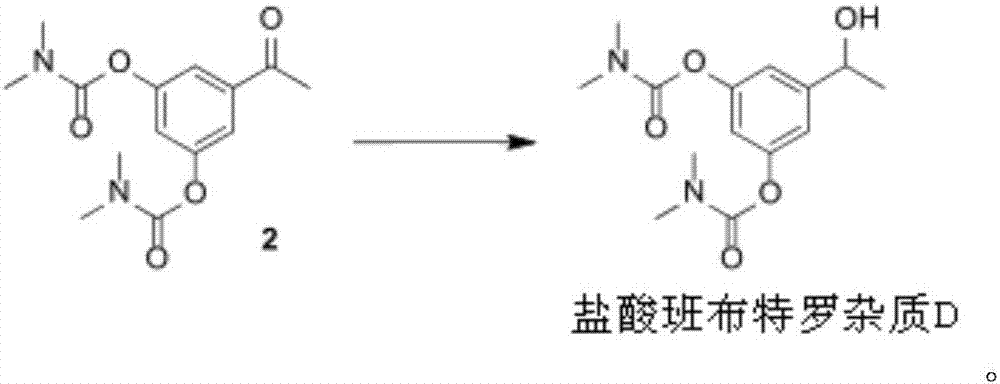
[0050] Example 2 Synthesis and preparation of bambuterol hydrochloride impurity D[5-(1-hydroxyethyl)-1,3-phenylene-bis(N,N-dimethylcarbamate)]
Embodiment 2-1
[0052] Take 3.5 grams of formula 2 compound [5-acetyl-1,3-phenylene-bis(N,N-dimethylcarbamate)] and dissolve it in 35 milliliters of anhydrous methanol, and reduce the temperature of the reaction system to 0- 5°C, then 1.45 g of NaBH 4 Add to the above solution several times. Stir at room temperature (25-30° C.) for 2 hours, concentrate to dryness under reduced pressure, add 80 ml of ethyl acetate and 50 ml of purified water, and separate and extract. The aqueous phase was extracted with ethyl acetate (50 ml*2), washed with water (100 ml*2), washed with saturated brine (100 ml), dried over anhydrous sodium sulfate for 2 hours, filtered, and washed with an appropriate amount of ethyl acetate, and the Concentrate under reduced pressure to obtain 3.1 grams of bambuterol hydrochloride impurity D[5-(1-hydroxyethyl)1,3-phenylene-bis(N,N-dimethylcarbamate)], yield: 88.0 %, the purity is 99%.
[0053] The characterization data of bambuterol hydrochloride impurity D[5-(1-hydroxyethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com