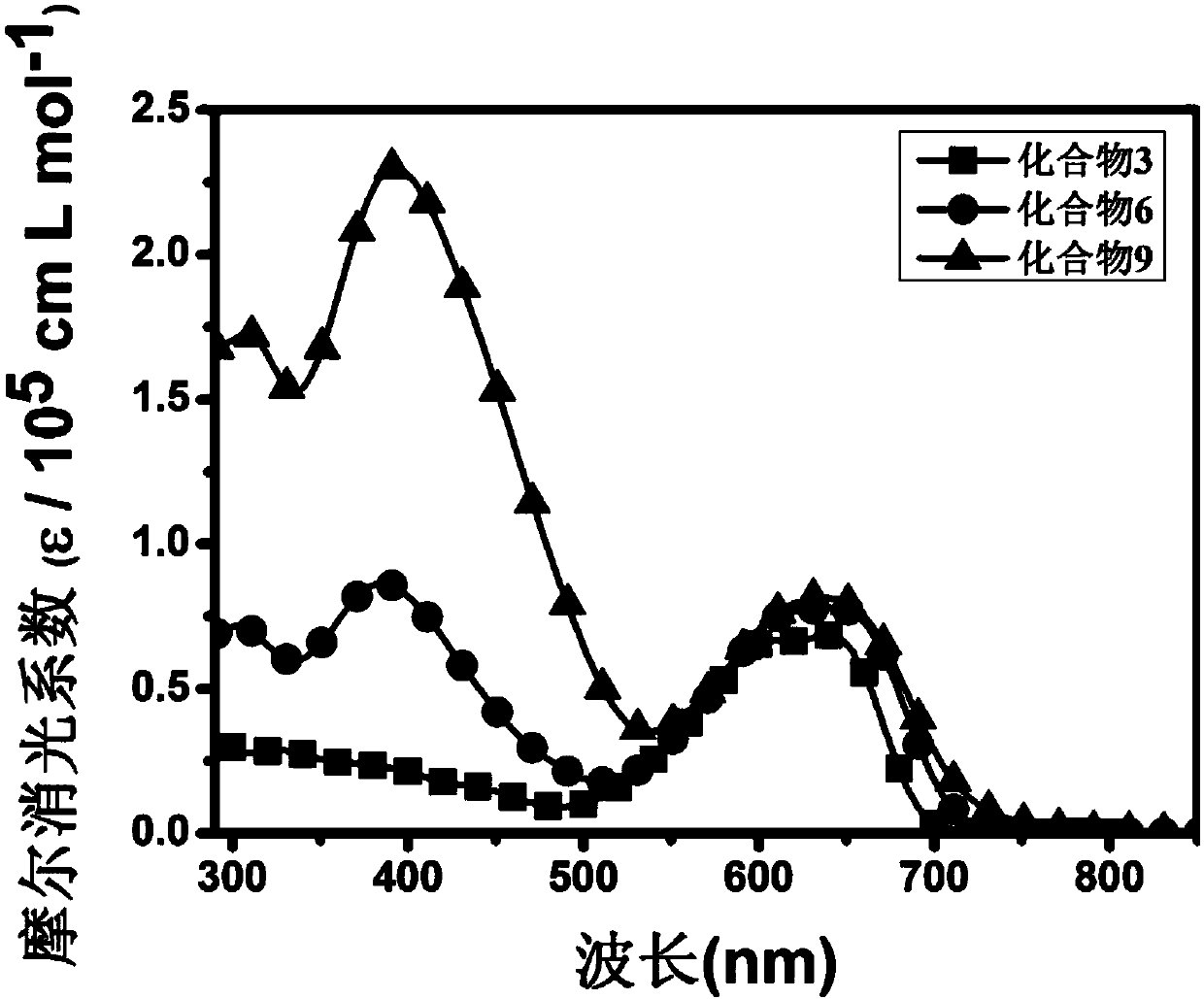
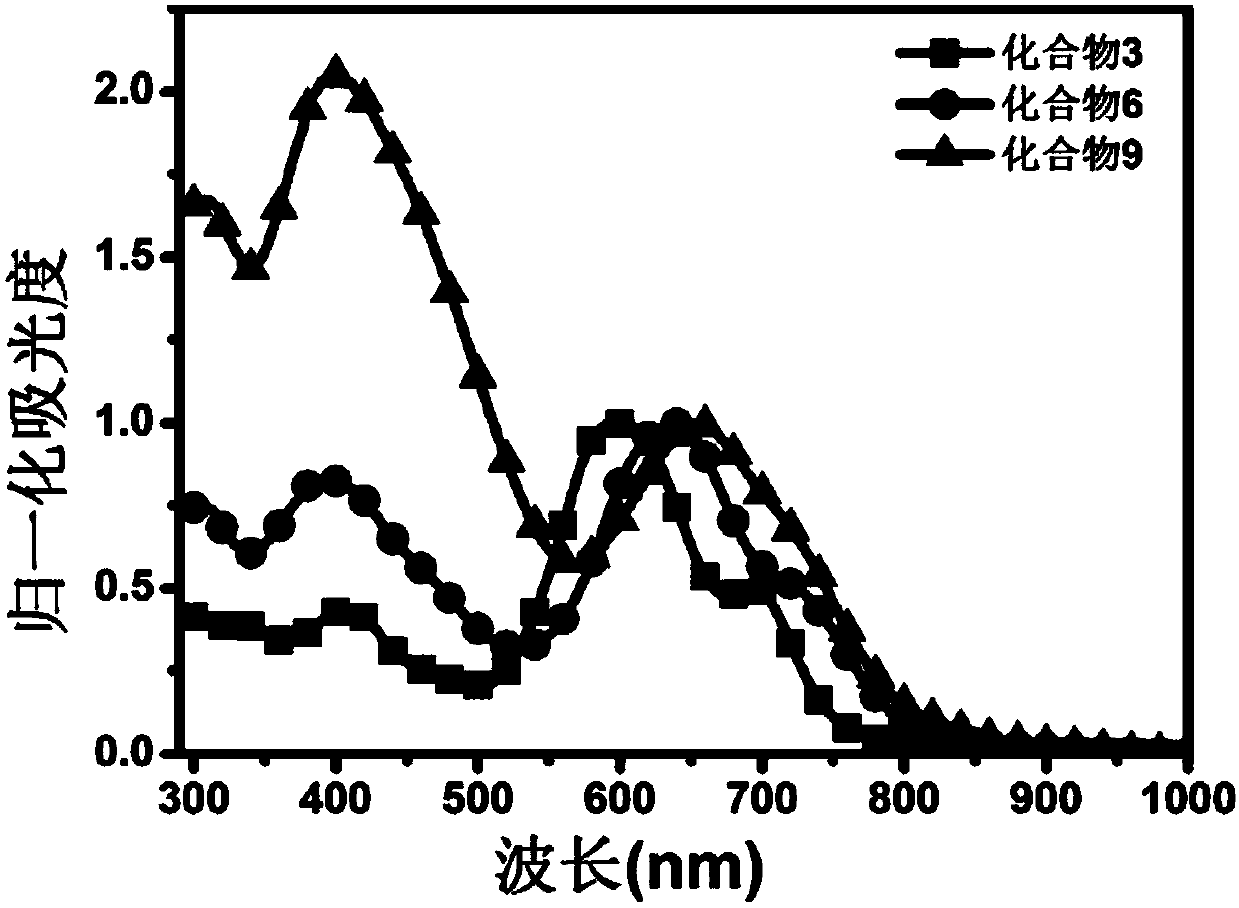
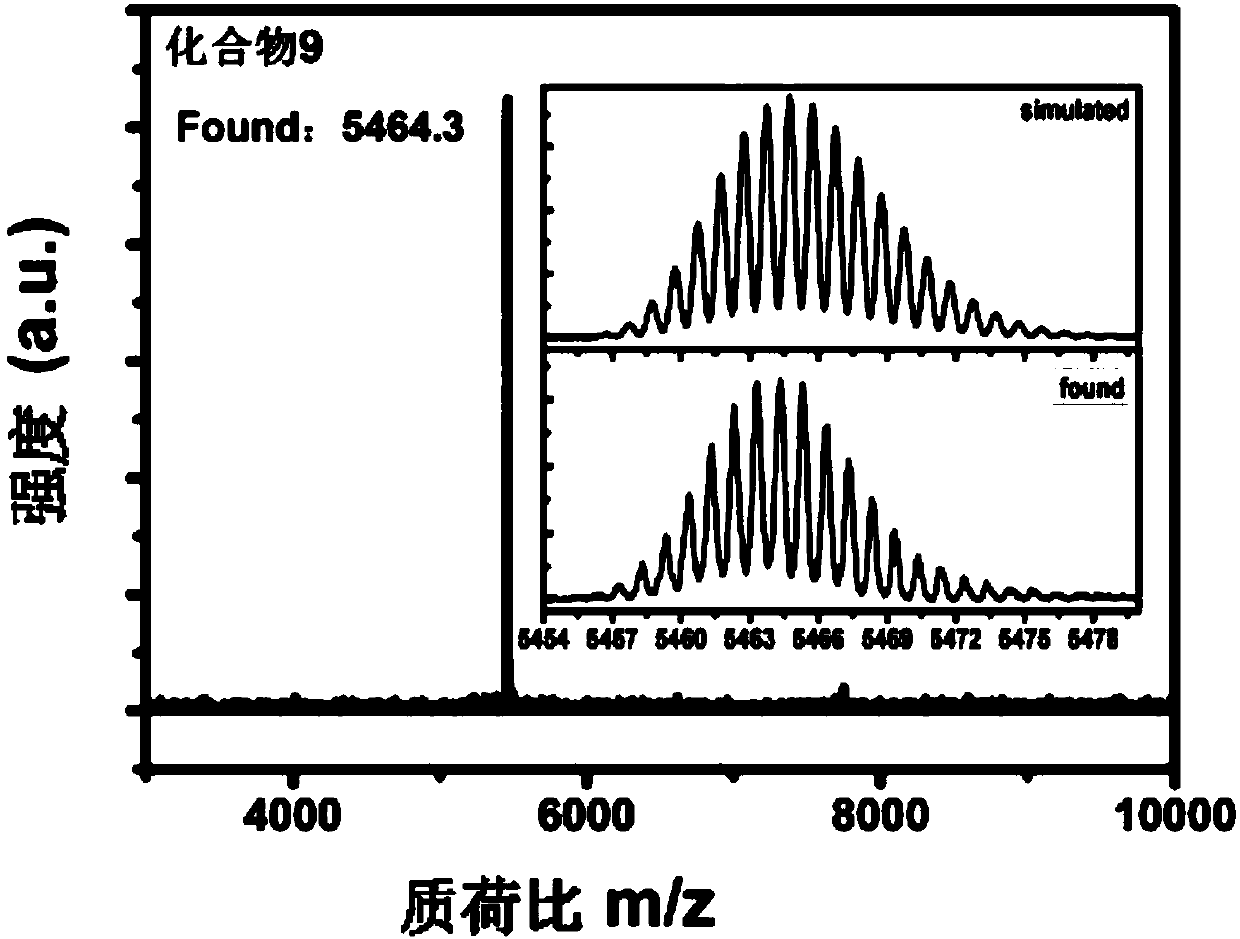
Functional modified branch-shaped conjugated compounds, ink, membranes and applications
A conjugated compound and conjugation technology, applied in the direction of silicon organic compounds, applications, compounds of group 4/14 elements of the periodic table, etc., can solve the problems of inability to fully utilize the solar spectrum and low collection capacity, and achieve the regulation of charge transfer The effect of reducing the spectral band gap and reducing the spectral bandwidth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1: Synthesis of compound 3
[0140]
[0141] Weigh compound 1 (1.0g, 984μmol), compound 2 (1.25g, 2.4mmol), Pd 2 (dba) 3 .CHCl 3 (120mg, 116μmol), HP(tBu) 3 BF 4 (70mg, 241μmol) was added to a 500mL two-necked round bottom flask under a nitrogen atmosphere, degassed THF (300mL) was added to the above two-necked flask, and then K was added dropwise to the reaction system 2 CO 3 Aqueous solution (1M, 15.0 mL, 15.0 mmol), stirring at room temperature overnight. The reaction system gradually turned blue. After 24 hours, first evaporate and concentrate most of the solvent in the solution, then extract the solution with dichloromethane / water, collect the organic phase, dry the organic phase with anhydrous sodium sulfate, filter, remove the solvent by rotary evaporation, and separate with a silica gel column The product, the eluent was dichloromethane: n-hexane (1:4), 1.4 g of blue solid compound 3 was obtained, and the yield was 88%. The characterization data are as fol...
Embodiment 2
[0143] Example 2: Synthesis of Compound 4
[0144]
[0145] The compound TBAF (270 mg, 855 μmol) was weighed and dissolved in 2 mL of THF to form a homogeneous solution. Take another 50mL reaction flask, weigh compound 3 (120mg, 73.2μmol) and dissolve it in 5mL THF, and dissolve it fully. 2ml of TBAF in THF was added dropwise to the compound 3 solution, and stirred at room temperature for 15 minutes. The reaction mixture was dropped into 30 ml of methanol, and the resulting precipitate was collected. The crude product obtained by precipitation was purified and separated by a silica gel chromatography column, and the eluent was dichloromethane: n-hexane (1: 3) to obtain 90 mg of compound 4 with a yield of 90%. The characterization data are as follows:
[0146] 1 H NMR(CDCl 3 ,400MHz):δ=8.92(d,J=3.80Hz;2H),7.32-7.33(m;8H),7.15-7.16(m;2H),7.10(m;2H),7.01-7.05(m;4H) ),4.04(d,J=7.76Hz;4H),1.98(br;2H),1.20-1.34(m;64H),0.82-0.87ppm(m;12H).MALDI-TOF MS:m / z calcd for C 78 H 100 N 2 O 2...
Embodiment 3
[0147] Example 3: Synthesis of Compound 6
[0148]
[0149] Weigh compound 1 (258mg, 254μmol), compound 5 (900mg, 780mmol), Pd 2 (dba) 3 .CHCl 3 (60mg, 57.9μmol), HP(tBu) 3 BF 4 (30mg, 104μmol) was added to a 250mL two-necked round bottom flask under a nitrogen atmosphere, degassed THF (135mL) was added to the above two-necked flask, and then K was added dropwise to the reaction system 2 CO 3 Aqueous solution (1M, 5.0 mL, 5.0 mmol), stirring at room temperature overnight. The reaction system gradually turned blue. After 24 hours, first evaporate and concentrate most of the solvent in the solution, then extract the solution with dichloromethane / water, collect the organic phase, dry the organic phase with anhydrous sodium sulfate, filter, remove the solvent by rotary evaporation, and separate with a silica gel column After removing small molecule by-products and catalysts. It was purified and separated again by gel permeation chromatography, using THF as the eluent to obtain 614 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com