Alkaline pectinase mutant with improved heat stability as well as encoding gene and application of alkaline pectinase mutant
A technology that encodes genes and pectinases, which can be used in applications, genetic engineering, plant genetic improvement, etc., can solve problems affecting the effect and decline of pectinases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1, the gene sequence preparation of alkaline pectinase PelN mutant
[0055] One, the preparation of the gene sequence of alkaline pectinase PelN single mutant
[0056] The wild-type alkaline pectinase PelN gene shown in sequence 1 is mutated to obtain the alkaline pectinase PelN mutant gene. The specific steps are as follows: the mutated nucleotides are introduced into the PelN gene by the method of segmented PCR amplification; the forward primer used in the front sequence PCR is the PelN universal primer PelN-F (the underlined part is the restriction site), and the reverse primer is the specific primer containing the mutation site (the part in bold is the mutated nucleotide), and the template is the PelN gene. The forward primer used in the subsequent sequence PCR is a specific primer containing a mutation site (the bold part is the mutated nucleotide), the reverse primer is the PelN general primer PelN-R (the underlined part is the restriction site), and t...
Embodiment 2
[0064] Embodiment 2, the preparation of alkaline pectinase PelN mutant
[0065] 1. Construction of expression vector
[0066] Insert the gene sequence of the wild-type alkaline pectinase PelN shown in sequence 1 between the Nde1 and Xho1 restriction sites of the expression vector pET-22b(+), and keep other sequences of the expression vector pET-22b(+) from The expression vector PelN is obtained; the expression vector PelN expresses the wild-type alkaline pectinase PelN with the HIS tag at the C-terminus, and the amino acid sequence of the wild-type alkaline pectinase PelN is sequence 6.
[0067] Insert the gene sequence of the alkaline pectinase PelN single mutant K93I shown in sequence 2 between the Nde1 and Xho1 restriction sites of the expression vector pET-22b(+), and keep the other parts of the expression vector pET-22b(+) The sequence remains unchanged, and the expression vector K93I is obtained; the expression vector K93I expresses the alkaline pectinase PelN mutant K9...
Embodiment 3
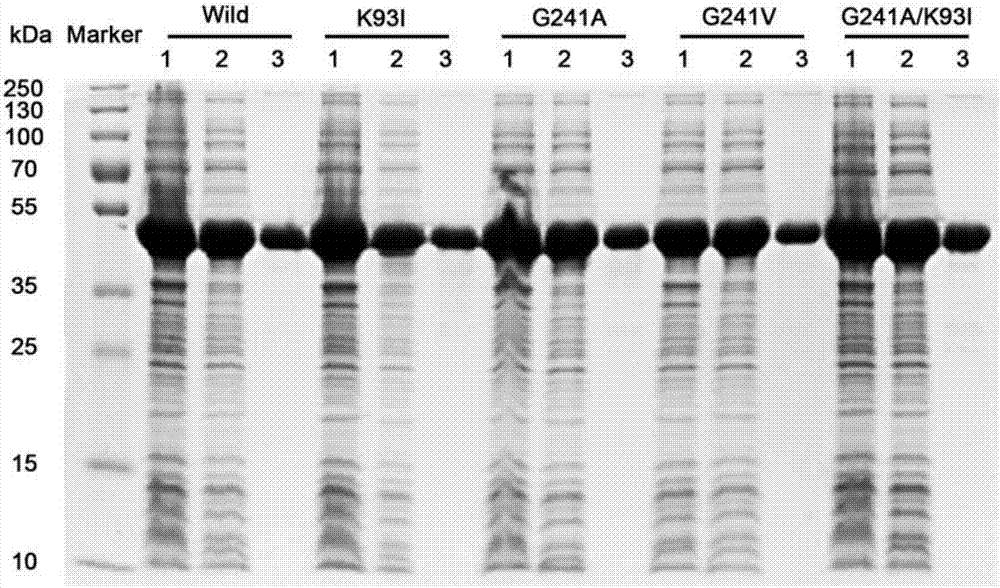
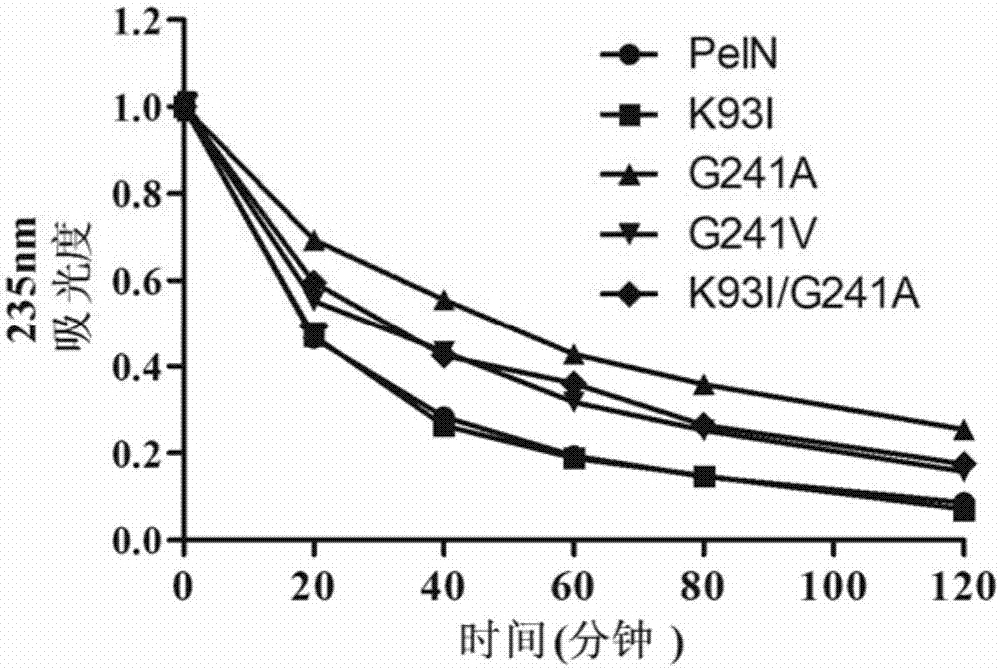
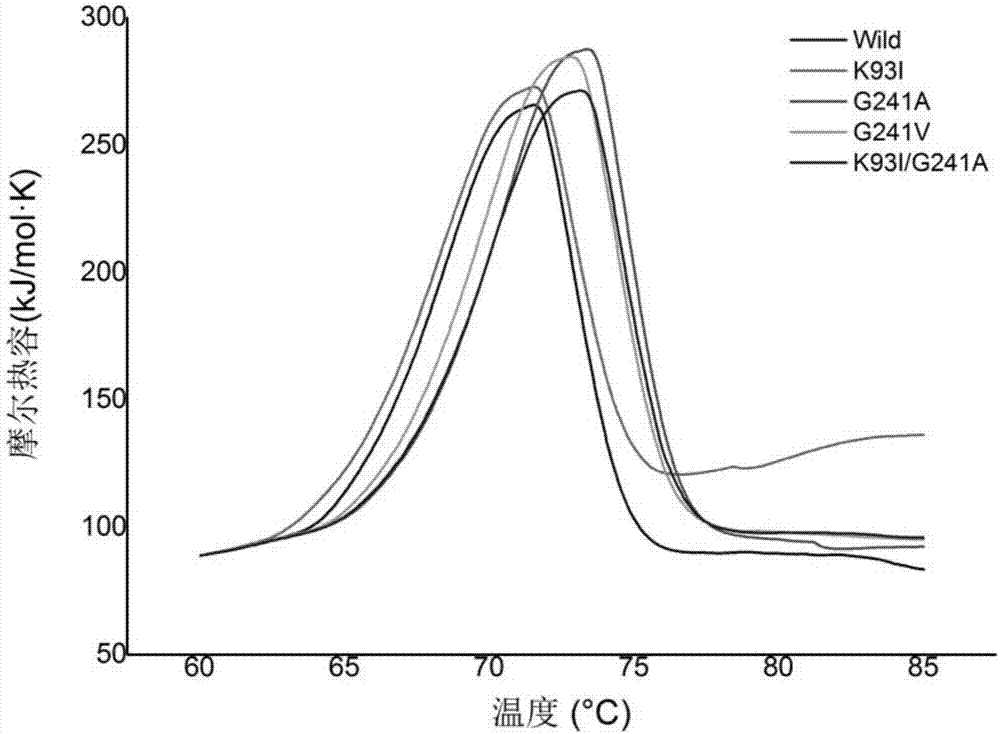
[0081] Embodiment 3, the thermostability comparison of wild-type alkaline pectinase PelN and mutant
[0082] The present invention compares wild-type alkaline pectinase PelN (PelN-WT, referred to as PelN or wild) and alkaline pectinase PelN single mutant K93I (PelN-K93I, referred to as K93I), alkaline pectinase PelN single mutant G241A (PelN-G241A, referred to as G241A), alkaline pectinase PelN single mutant G241V (PelN-G241V, referred to as G241V) and alkaline pectinase PelN double mutant K93I / G241A (PelN-K93I / G241A, referred to as K93I / G241A), wherein the wild-type alkaline pectinase PelN is Paenibacillus sp.0602 (GenBank accession number: KC351190) derived alkaline pectinase PelN-WT (amino acid sequence is sequence 6, which encodes The gene sequence is sequence 1).
[0083] 1. Comparison of remaining enzyme activity, half-life and specific activity of wild-type alkaline pectinase PelN and mutants after treatment at 60°C for different times
[0084] 1. Residual enzyme acti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com