Method for preparing ethyl methyl carbonate through ester exchange method
A technology of ethyl methyl carbonate and dimethyl carbonate, applied in the field of preparing chemical raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The prepared Al with composite pore structure 2 o 3 -SiO 2 200 g of the carrier was placed in a muffle furnace and calcined at 500 °C for 4 hours to remove Al 2 o 3 -SiO 2 Adsorbed water; take 0.75 mol (192 g) Mg(NO 3 ) 2 ·6H 2 O, 0.10 mol (21.4 g) MgCl 2 ·6H 2 O was dissolved in 1000 mL deionized water, and the prepared mixed solution of magnesium nitrate and magnesium chloride was impregnated into the calcined Al 2 o 3 -SiO 2 In the carrier channel; the impregnated catalyst precursor was dried in an oven at 120 °C for 10 h; the dried catalyst precursor was calcined in a muffle furnace at 600 °C for 4 h to obtain a MgO loading of 15 % wt and a MgCl loading A supported catalyst with composite pore structure 15% MgO-5% MgCl in the amount of 5% wt 2 / Al 2 o 3 -SiO 2 .
[0070] The catalyst support was replaced by SiO 2 -SiO 2 、Al 2 o 3 -SiO 2 , SiO 2 -Al 2 o 3 、Al 2 o 3 -Al 2 o 3 , single macroporous SiO 2 , single small hole SiO 2 , using a ...
Embodiment 2
[0076] The prepared Al with composite pore structure 2 o 3 -SiO 2 200 g of the carrier was placed in a muffle furnace and calcined at 500 °C for 4 hours to remove Al 2 o 3 -SiO 2 Adsorbed water; take 0.75 mol (192 g) Mg(NO 3 ) 2 ·6H 2 O, 0.10 mol (21.4 g) MgCl 2 ·6H 2 O, 0.025 mol (8.42g) La(NO 3 ) 3 ·H 2 O was dissolved in 1000 mL deionized water, and the prepared mixed solution of magnesium nitrate, magnesium chloride and lanthanum nitrate was impregnated into the calcined Al 2 o 3 -SiO 2 In the carrier channel; the impregnated catalyst precursor was dried in an oven at 120 °C for 10 h; the dried catalyst precursor was calcined in a muffle furnace at 600 °C for 4 h to obtain a MgO loading of 15% wt, MgCl 2 With a loading of 5% wt, La 2 o 3 Supported catalyst 15%MgO-5%MgCl with multi-active centers and composite pore structure with a loading of 2% wt 2 -2%La 2 o 3 / Al 2 o 3 -SiO 2 .
[0077] In the fixed-bed reactor, load 50 g of the catalyst prepared...
Embodiment 3
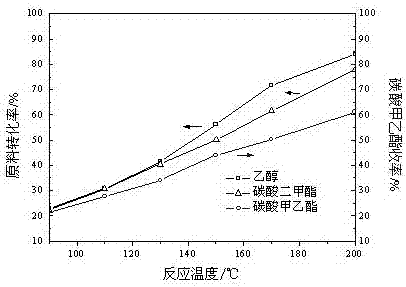
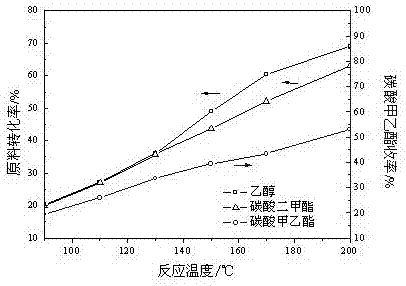
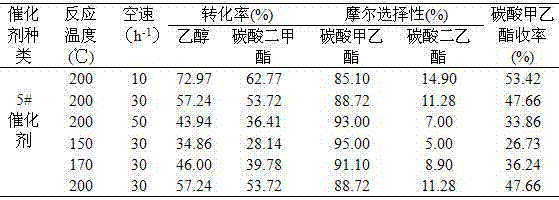
[0082] In the fixed-bed reactor, 50 g of catalysts prepared in Example 2 were loaded, and dimethyl carbonate and ethanol were pumped into the reactor according to the molar ratio of 1:1 by using a constant flow pump, and the space velocity was 10 respectively. h -1 、20 h -1 、30 h -1 、40 h -1 and 50h -1 , normal pressure, the reaction temperature was 200°C, and it was stabilized for 500 h. After stabilization at different space velocities, samples were taken for chromatographic analysis and calculation. The conversion rate of raw materials, product selectivity and product yield are shown in Table 3.
[0083] Table 3 Effects of different space velocities on raw material conversion, product selectivity and product yield
[0084]
[0085] As can be seen from Table 3, with the increase of space velocity, the conversion rate of raw materials and the yield of ethyl methyl carbonate decreased gradually. The smaller the mass space velocity, the longer the residence time of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com