A method for continuously preparing azo dye in micro-reactors
A micro-reactor, azo dye technology, applied in the direction of azo dyes, monoazo dyes, chemical instruments and methods, etc., can solve the problem that the diazotization reaction temperature is difficult to control, the yield of diazonium salts is not high, and the reaction cycle To avoid decomposition and other side reactions, shorten the residence time, and shorten the reaction period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
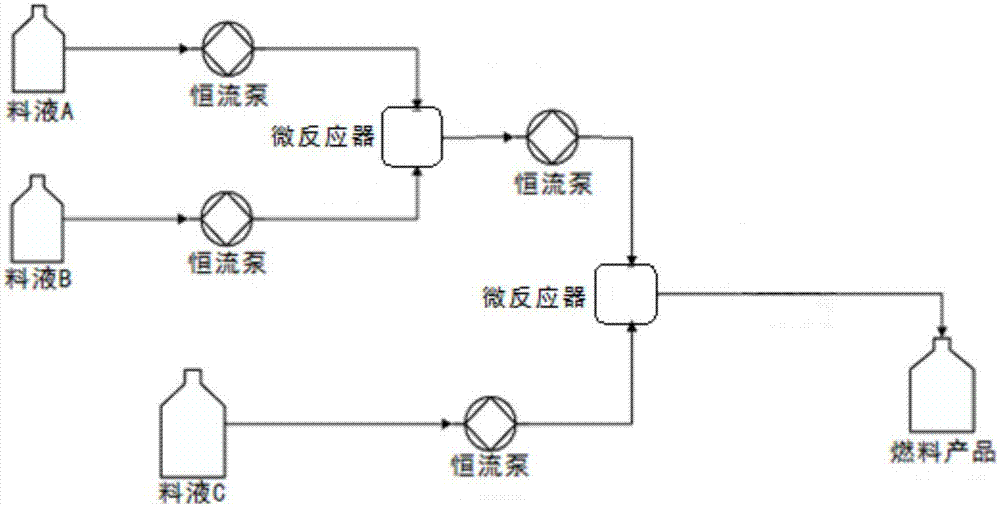
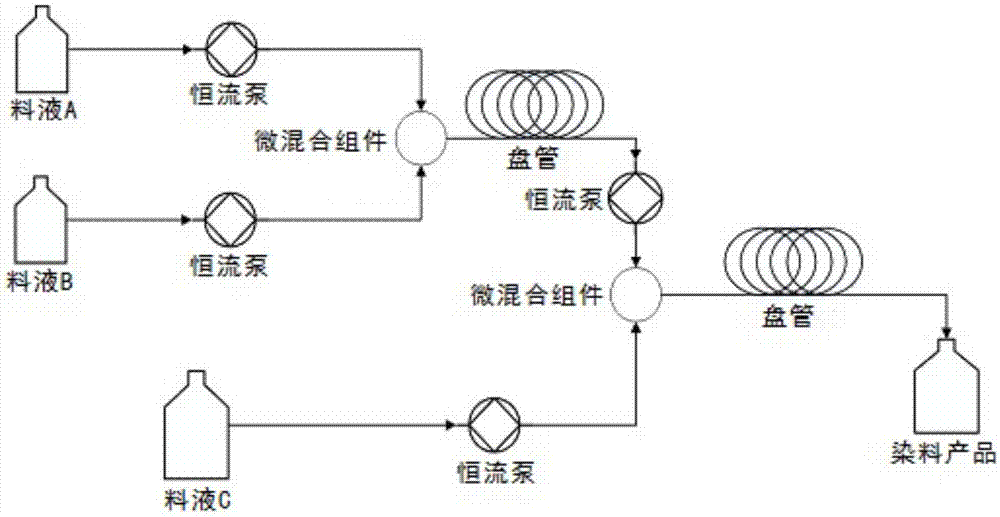
[0030] Embodiment 1: the concrete steps of continuous preparation azo dye product 1# are as follows (as figure 1 and figure 2 ):
[0031] (1) At room temperature (25°C), take 0.930g of aniline in a 100mL beaker, add 15mL of DMF (N,N-dimethylformamide), 2.5mL of concentrated hydrochloric acid (37%), and 32.5mL of deionized water, Stir evenly to obtain material liquid A;
[0032] (2) Take 0.731g of solid sodium nitrite in a 100mL beaker, add 15mL of DMF (N,N-dimethylformamide), 35mL of deionized water, stir until the solid of sodium nitrite is completely dissolved, and obtain feed liquid B;
[0033] (3) At room temperature (25°C), take 2.54 g of the coupling component 1-(4-sulfophenyl)-3-methyl-5-pyrazolone in a beaker, add 60 mL of water, 30 mL of DMF ( N,N-dimethylformamide) and shake well, add sodium hydroxide solution to adjust the pH of the coupling component to 9.95-10.05, stir until the coupling component is completely dissolved, and obtain material liquid C;
[0034...
Embodiment 2
[0037] Embodiment 2: the concrete steps of continuous preparation azo dye product 2-12# are as follows (as figure 1 and figure 2 ):
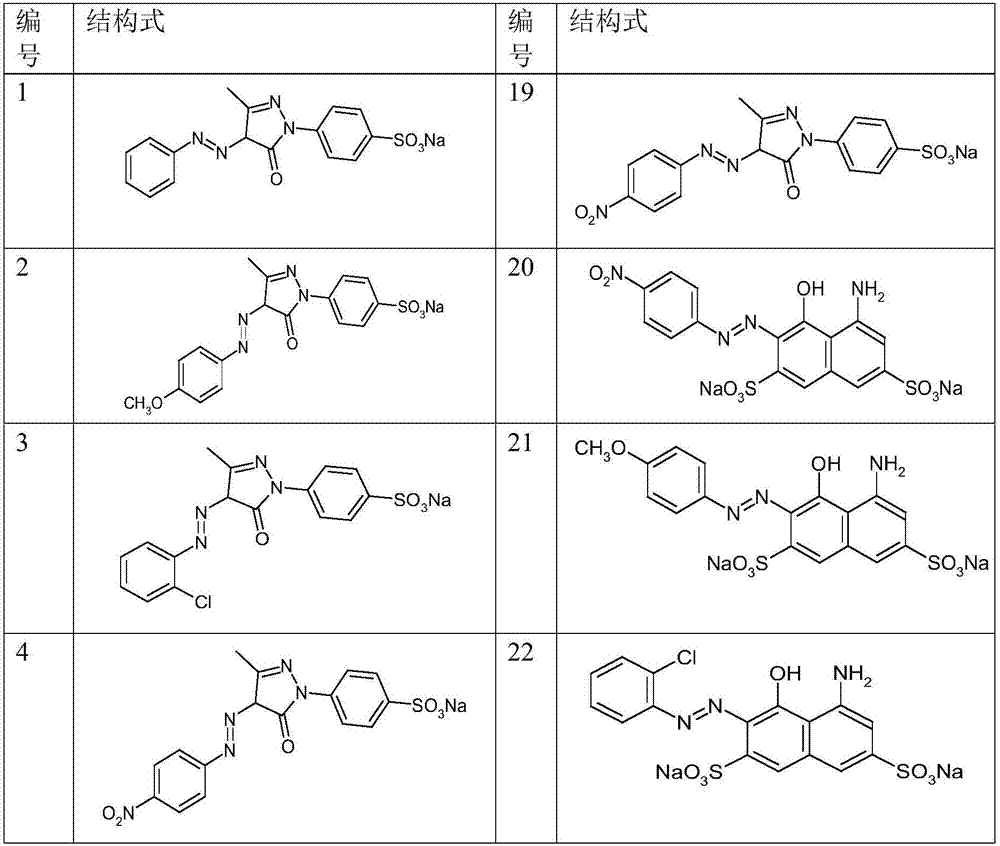
[0038] Use p-methoxyaniline, o-chloroaniline, p-nitroaniline, m-nitroaniline, o-nitroaniline, 1-naphthylamine, p-cyanoaniline, N, N-dimethylaniline, p-aminobenzenesulfonic acid , o-aminobenzenesulfonic acid, 1-amino-4-naphthalenesulfonic acid instead of the diazo component aniline in Example 1, and coupling component 1-(4-sulfonic acid phenyl)-3-methyl-5 -Pyrazolone is used for the continuous synthesis of azo dyes, and other conditions are consistent with Example 1 to obtain the azo dyes of 2-12# structure (see Table 1 for the structural formula).
Embodiment 3
[0039] Embodiment 3: the concrete steps of continuous preparation azo dye product 13# are as follows (as figure 1 and figure 2 ):
[0040] (1) At room temperature (25°C), put 0.930kg of aniline into a 100L container, add 15LDMF (N,N-dimethylformamide), 2.5L concentrated hydrochloric acid (37%), and 32.5L deionized water, and stir Evenly, the material liquid A is obtained;
[0041] (2) Take 0.731kg of solid sodium nitrite in a 100L container, add 15LDMF (N,N-dimethylformamide) and 35L of deionized water, and stir until the solid sodium nitrite is completely dissolved to obtain feed liquid B;
[0042] (3) At room temperature (25°C), take 3.68kg of monosodium H acid, the coupling component, into a container, add 60L of water, 30LDMF (N,N-dimethylformamide) and shake well, then add sodium hydroxide solution Adjust the pH of the coupling components to 9.95-10.05, stir until the coupling components are completely dissolved, and obtain feed liquid C;
[0043] (4) The micro-react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com