Icariin sustained and controlled release nano-particle and method for preparing same
An icariin and nanoparticle technology is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., which can solve the problems of low bioavailability, poor oral absorption, Difficulty and other problems, to achieve the effect of good repeatability, improved solubility, simple and cheap materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
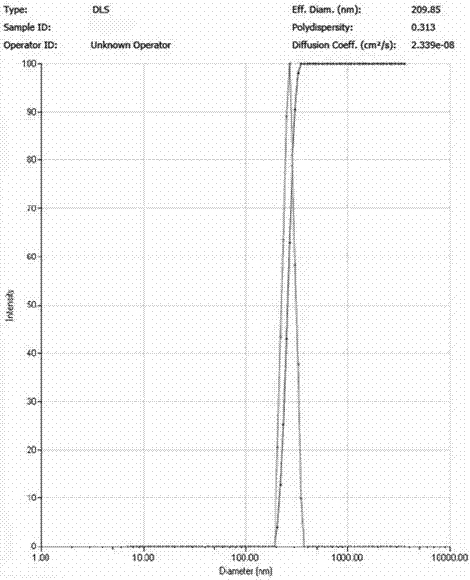
[0024] Dissolve 10 mg of icariin raw material drug and 30 mg of PLGA in 10 ml of methanol / dichloromethane solution (1:1 volume ratio), and prepare an icariin / PLGA solution with a concentration of 1.0 mg / ml; Dissolve 300 mg of PVA in 10 ml of water to prepare a PVA solution with a concentration of 30 mg / ml. Measure 0.75 ml of icariin / PLGA solution and add it to 2.25 ml of PVA solution, and then perform ultrasonic emulsification on the mixture; the ultrasonic frequency is 20-25 KHz, the power is 250 W, the ultrasonic time is 6 min, and each ultrasonic wave is 5 s , with an interval of 10 s. The emulsified solution was transferred to a rotary evaporator, and the methanol, dichloromethane, and chloroform were completely evaporated by rotary evaporation at room temperature for 10 minutes, and then the sample was centrifuged at high speed for 30 minutes (4°C, 20,000 rpm) to collect the precipitate and add Resuspend in deionized water and centrifuge again. The operation was repeate...
Embodiment 2
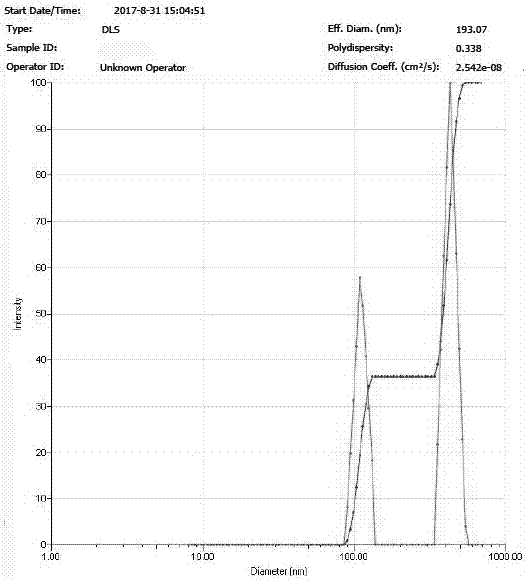
[0027] Dissolve 20 mg icariin raw material drug and 200 mg PLGA in 10 ml methanol / dichloromethane solution (volume ratio 5:1), and prepare icariin / PLGA solution with a concentration of 2.0 mg / ml; Dissolve 300 mgPVA in 10 ml of water to prepare a PVA solution with a concentration of 30 mg / ml. Measure 0.75 ml of icariin / PLGA solution and add it to 2.25 ml of PVA solution, and then carry out ultrasonic emulsification of the mixture; the ultrasonic frequency is 20-25 KHz, the power is 100 W, the ultrasonic time is 6 min, every ultrasonic 5 s, The interval is 10 s. The emulsified solution was transferred to a rotary evaporator, and the methanol, dichloromethane, and chloroform were completely evaporated by rotary evaporation at room temperature for 10 minutes, and then the sample was centrifuged at high speed for 30 minutes (4°C, 20,000 rpm) to collect the precipitate and add Resuspend in deionized water and centrifuge again. The operation was repeated 5 times, and the nanopartic...
Embodiment 3
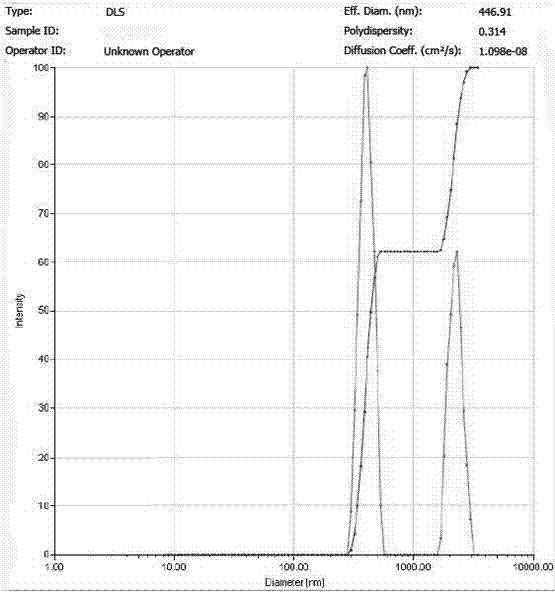
[0029] Dissolve 30 mg icariin raw material drug and 10 mg PLGA in 10 ml methanol / ethyl acetate solution (volume ratio 9:1), and prepare icariin / PLGA solution with a concentration of 3.0 mg / ml; Dissolve 300 mgPVA in 10 ml of water to prepare a PVA solution with a concentration of 30 mg / ml. Measure 0.75 ml of icariin / PLGA solution and add it to 2.25 ml of PVA solution, and then carry out ultrasonic emulsification of the mixture; the ultrasonic frequency is 20-25 KHz, the power is 400 W, the ultrasonic time is 6 min, every ultrasonic 5 s, The interval is 10 s. The emulsified solution was transferred to a rotary evaporator, and the methanol, dichloromethane, and chloroform were completely evaporated by rotary evaporation at room temperature for 10 minutes, and then the sample was centrifuged at high speed for 30 minutes (4°C, 20,000 rpm) to collect the precipitate and add Resuspend in deionized water and centrifuge again. The operation was repeated 5 times, and the nanoparticles...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com