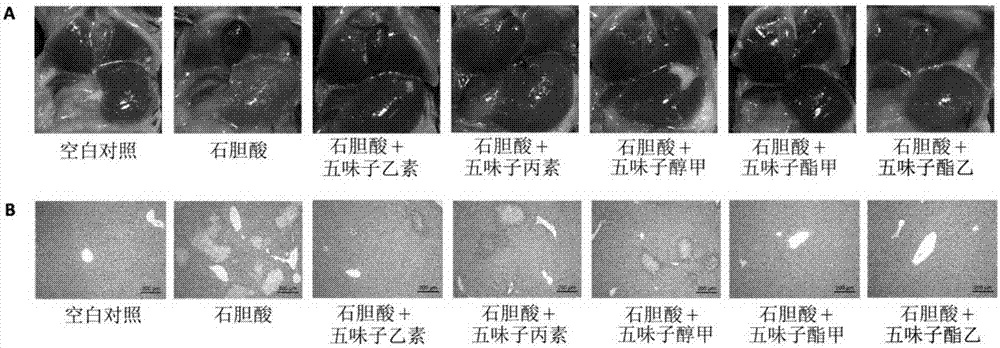
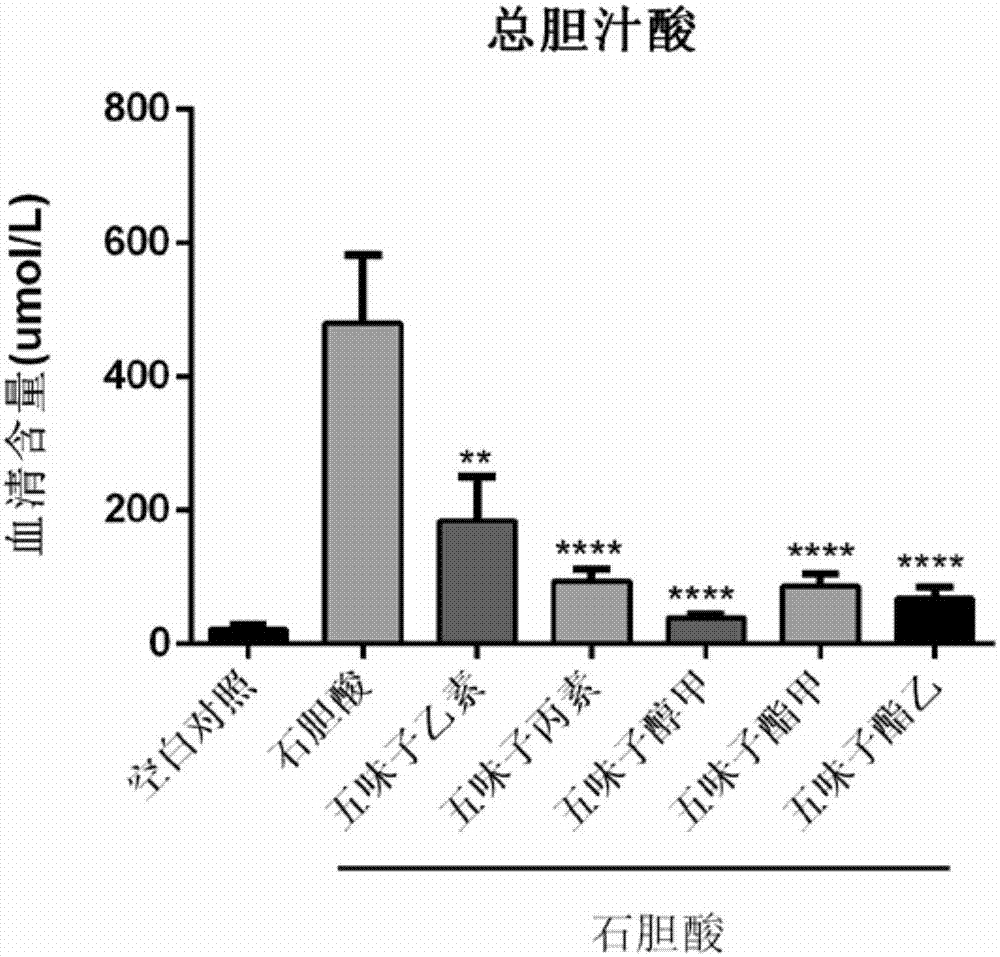
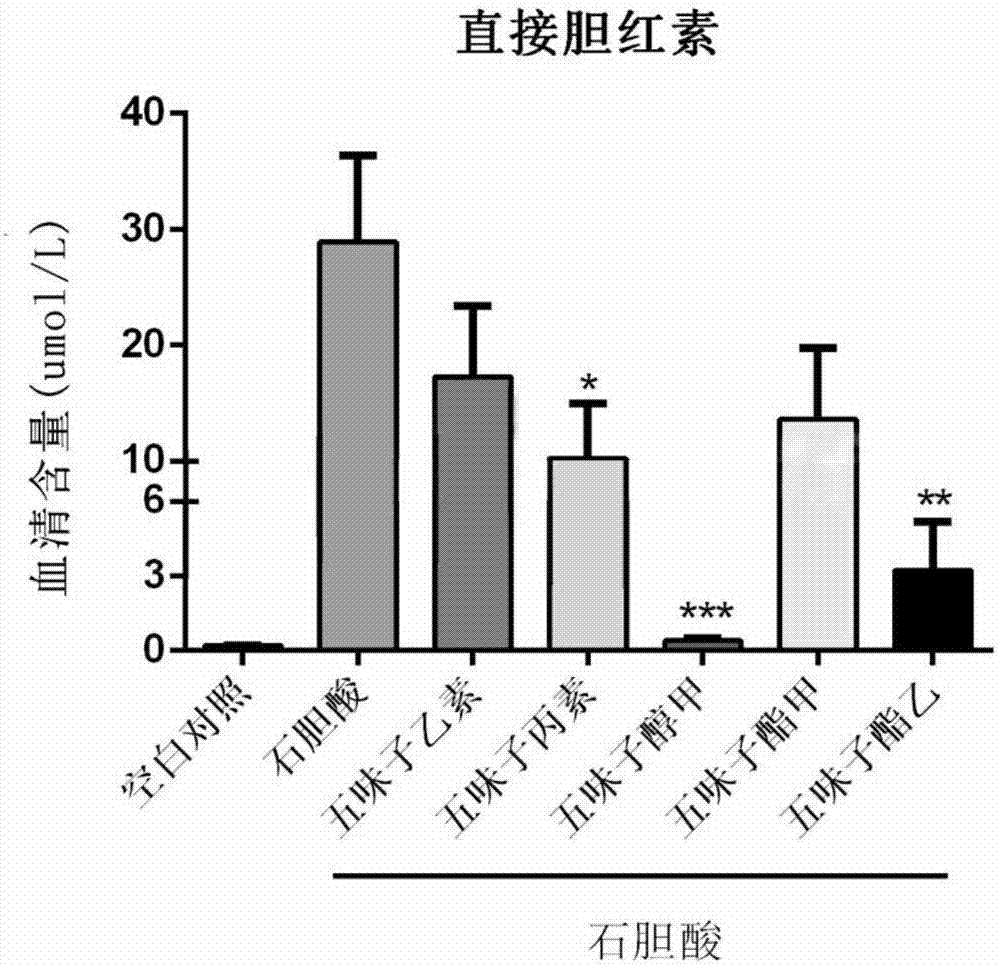
Application of monomeric compounds of fruits of Chinese magnoliavine (Schisandra chinensis(Turcz.) baill. and Schisandra sphenanthera Rehd.et Wils) to preparation of medicine for preventing and treating cholestasis liver diseases
A technology for cholestasis and Schisandra chinensis, applied in the field of medicine, can solve the problems of limited content, restrict the full development and application of Schisandra chinensis, and achieve the effect of broadening the scope of application and good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Prevention and treatment of mouse intrahepatic cholestasis model caused by excessive lithocholic acid of embodiment 1
[0037] 1. Materials and instruments
[0038] (1) Main instruments:
[0039] 5417-R low-temperature high-speed centrifuge (Eppendorf, Germany); full-wavelength microplate reader (Thermo, USA); Hitachi 7020 automatic biochemical analyzer (HITACHI, Japan)
[0040] (2) Drugs and reagents:
[0041] Table 1 The source and dosage of the main reagents used in the test
[0042]
[0043]
[0044] 2. Experimental method
[0045] (1) Experimental animals
[0046] C57BL / 6 mice, male, 7-9 weeks old, were provided by the Guangdong Medical Experimental Animal Center, license number SYXK (Guangdong) 2016-0112, and normal mice were fed with maintenance feed. All mice were randomly divided into 7 groups, 7 to 9 in each group, the groups were: blank control group, LCA model group, SinB+LCA group, SinC+LCA group, SolA+LCA group, StnA+LCA group, StnB+LCA group g...
Embodiment 2
[0070] A pharmaceutical composition for preventing and treating cholestatic liver disease, which comprises one or more of Schizandrin B, Schizandrin C, Schisandrin A, Schizandrin A or Schizandrin B.
[0071] The medicine can be prepared into tablets, capsules, granules, powders, solutions or suspensions by adding auxiliary materials. Simultaneously, the dosage form of the medicine can also be made into a sustained-release agent or a controlled-release agent according to actual needs.
[0072]Wherein, the auxiliary material is one or more of fillers, binders, wetting agents, disintegrants, flavoring agents, lubricants or preservatives.
[0073] Specifically, the filler can be selected from one or more of starch, sucrose, dextrin, lactose, microcrystalline cellulose or mannitol; the wetting agent and binder can be selected from starch slurry, fiber One or more of vegan derivatives, alginate, gelatin or glycerin; the disintegrant can be selected from dry starch, sodium starch gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com