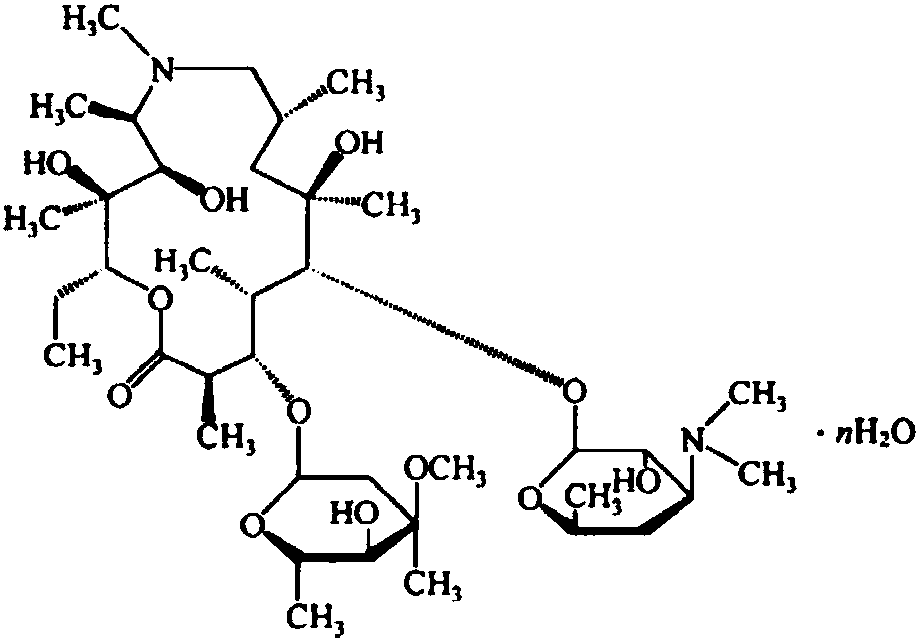
Azithromycin capsule and preparation method thereof
A technology of azithromycin and capsules, which is applied in the field of pharmaceutical preparations, can solve the problems of fragmentation, large difference in weight, and poor powder fluidity, and achieve the effects of high yield, less over-grinding, and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Take by weighing 1.309kg of azithromycin raw material, 0.266kg of microcrystalline cellulose, 0.079kg of crospovidone, and 0.029kg of magnesium stearate, mix with a three-dimensional mixer for 5min, granulate with a dry granulator, and weigh 0.0045kg Sodium lauryl sulfate is mixed with the granules for 3 minutes, and the filling agent is prepared. Pass through a 20-mesh sieve to obtain granules. The granules have good fluidity. Fill gelatin capsules with No. 1 gelatin, and control the filling volume to 340 mg.
[0016] name
Embodiment 2
[0018] Take by weighing 1.311kg of azithromycin raw material, 0.265kg of microcrystalline cellulose, 0.077kg of crospovidone, and 0.029kg of magnesium stearate, mix with a three-dimensional mixer for 5min, granulate with a dry granulator, and weigh 0.005kg Sodium lauryl sulfate is mixed with the granules for 3 minutes, and the filling agent is prepared. Pass through a 20-mesh sieve to obtain granules. The granules have good fluidity. Fill gelatin capsules with No. 1 gelatin, and control the filling volume to 340 mg.
Embodiment 3
[0020] Take by weighing 1.309kg of azithromycin raw material, 0.267kg of microcrystalline cellulose, 0.074kg of crospovidone, and 0.029kg of magnesium stearate, mix with a three-dimensional mixer for 5min, and granulate with a dry granulator, weighing 0.0042kg Sodium lauryl sulfate is mixed with the granules for 3 minutes, and the filling agent is prepared. Pass through a 20-mesh sieve to obtain granules. The granules have good fluidity. Fill gelatin capsules with No. 1 gelatin, and control the filling volume to 340 mg.
[0021] Described microcrystalline cellulose can adopt any one in PH-101, PH-102, PH-103, PH-105, PH-F20, PH-301, PH-302, KG-801, preferably PH302, microcrystalline fiber Prime 302 is free-flowing extremely fine short rod-like or powdery particles, white or near-white in color, odorless, tasteless, and has the characteristics of good fluidity, rapid disintegration and dissolution. The above-mentioned microcrystalline cellulose PH-302 It is an excellent new typ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com