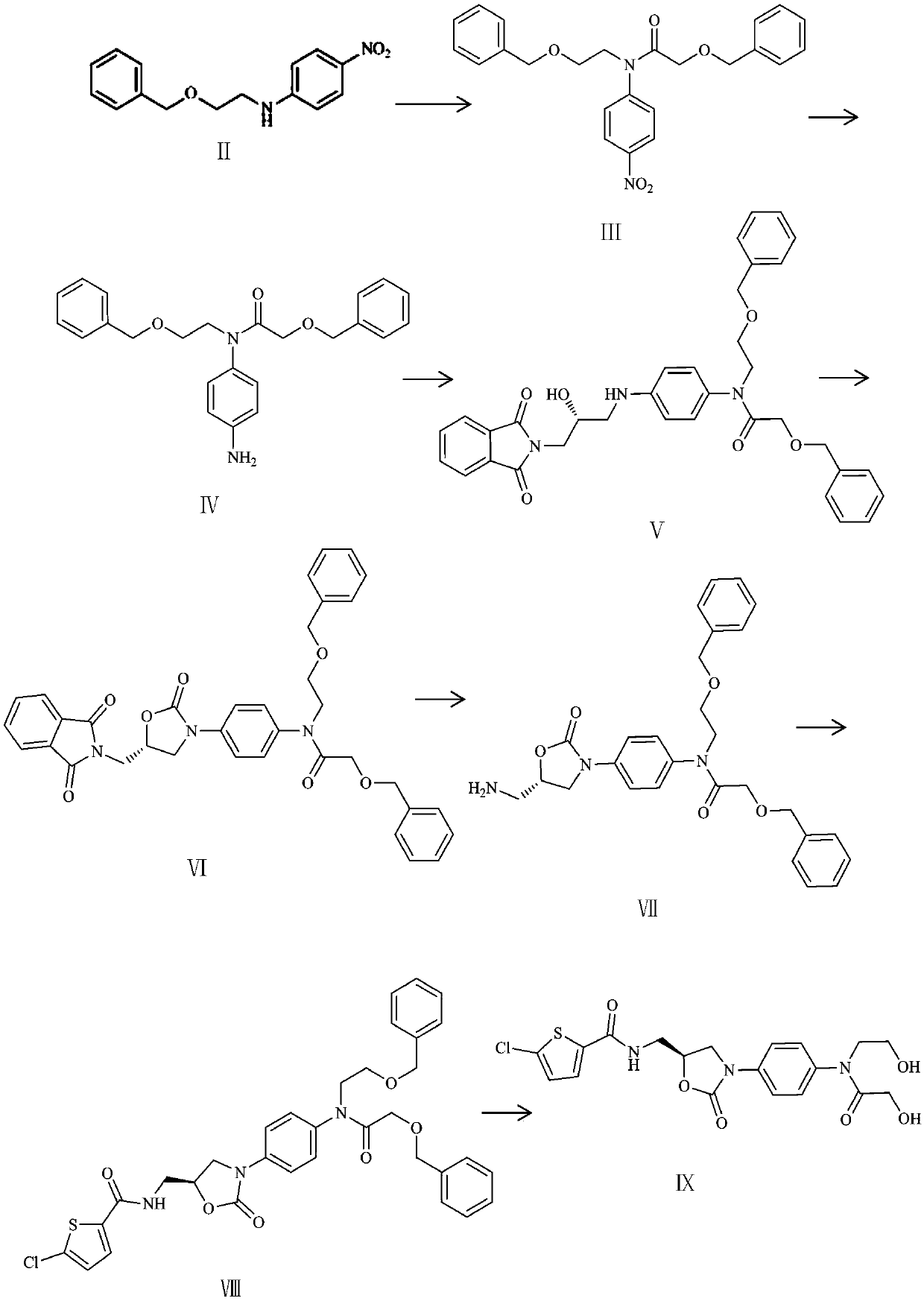
Synthetic method of rivaroxaban metabolite 5
A synthetic method and technology of rivaroxaban, which is applied in the field of synthesis of rivaroxaban metabolite 5, can solve the problems that metabolite research has not yet been reported, and achieve a high operability, reasonable process design and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
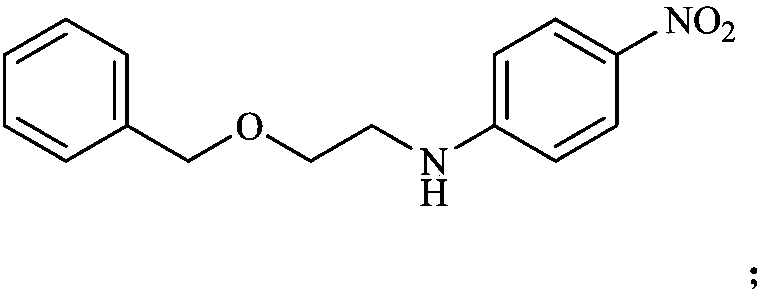
[0030] (1) Dissolve 10 grams of p-bromonitrobenzene in 50 milliliters of acetonitrile, add 6.5 grams of 2-benzyloxyethylamine to the above solution under ice cooling, then add 20 milliliters of triethylamine, and react at room temperature for 6 hours to obtain yellow Clarify the solution, concentrate the reaction solution under reduced pressure, dissolve it with 200 milliliters of dichloromethane, wash three times with 50 milliliters of water, combine the organic phases, dry with anhydrous sodium sulfate, spin dry after filtration to obtain the crude product, and purify the crude product by column chromatography 10.8 compounds II were obtained with a yield of 91.7%.
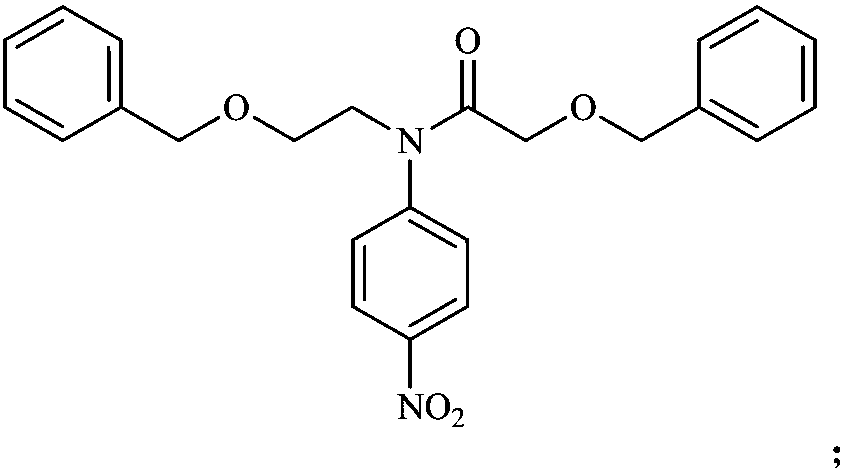
[0031] (2) Dissolve 10.8 grams of intermediate II in 250 milliliters of dichloromethane, add 7.67 grams of benzyloxyacetic acid, 26.33 grams of HATU (2-(7-benzotriazole oxide)-N,N, N',N'-tetramethylurea hexafluorophosphate) and 19 ml of N,N-diisopropylethylamine, and then reflux at 40°C for 24 hours. The reactio...
Embodiment 2
[0039] (1) Dissolve 5 grams of p-fluoronitrobenzene in 50 milliliters of acetonitrile, add 5.72 grams of 2-benzyloxyethylamine (A) to the above solution under ice-cooling, then add 9.5 milliliters of triethylamine, and react at 50°C for 8 Hours, a yellow clear solution was obtained, the reaction solution was concentrated under reduced pressure, dissolved with 200 ml of dichloromethane, washed three times with 50 ml of water, the organic phases were combined, dried with anhydrous sodium sulfate, filtered and spin-dried to obtain a crude product. The crude product was purified by column chromatography to obtain 4.5 g of intermediate II as a yellow oil with a yield of 48.1%.
[0040] (2) Dissolve 2 g of intermediate II in 20 ml of dry dichloromethane, add 1.7 ml of dry pyridine under ice-cooling, then dissolve 1.2 g of benzyloxyacetyl chloride in 5 ml of dry dichloromethane, slowly dropwise add The reaction solution was then reacted at 0°C for 5 hours. The reaction solution was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com