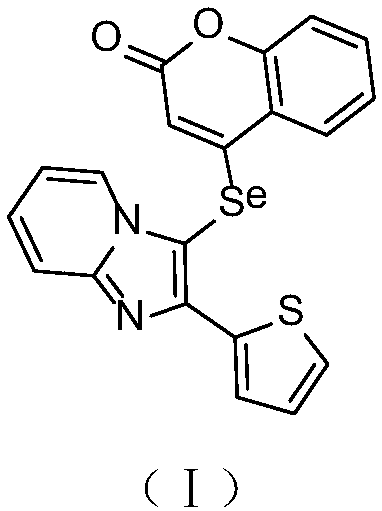
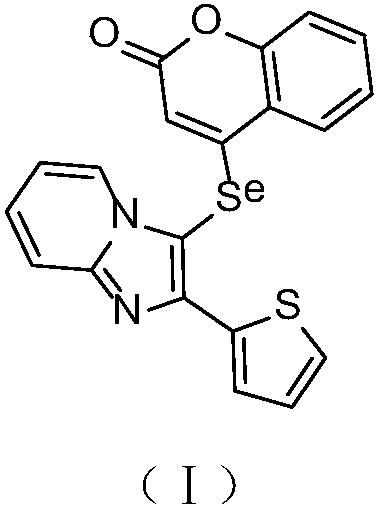
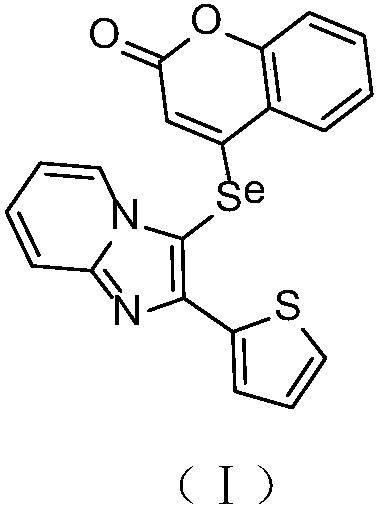
Synthesis and application of imidazole selenium-containing pyrone compound
A compound, pyrone technology, applied in the fields of organic chemistry, drug combination, anti-tumor drugs, etc., can solve the problems of toxicity, humidity, expensive and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Step 1: After dissolving 4-hydroxycoumarin (1mmol) and triethylamine (1.5mmol) in 10mL of dichloromethane, cooling in a cold water bath, trifluoromethanesulfonic anhydride (1.5mmol) was slowly added dropwise to the reaction system, and the reaction After 3 hours, the solvent was removed by rotary evaporation, concentrated and purified by silica gel column separation (volume ratio of petroleum ether to ethyl acetate: 4:1) to obtain 4-trifluoromethanesulfonate-coumarin.
[0015] Step 2: Under the protection of oxygen, add 2-aminopyridine (0.5mmol), 2-thiophenethanone (10.0mmol), cuprous iodide (0.25mmol) and boron trifluoride diethyl ether (0.14mL) into 2mL of DMF solvent , reacted at 60°C for 12 hours; cooled to room temperature, extracted with ethyl acetate (30mL), dried over anhydrous sodium sulfate, and purified by concentrated silica gel column chromatography (the volume ratio of petroleum ether to ethyl acetate was 3:1) to obtain 2-(2-(Thienimidazo)[1,2-a]pyridine. ...
Embodiment 2
[0025] Activity determination of the above compounds
[0026] Experimental Materials:
[0027] 1. Human cancer cell lines: all were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences.
[0028] 2. The drug under test: After dissolving in DMSO, prepare it to an initial concentration of 10,000 mg / mL for later use.
[0029] 3. 0.9% normal saline: 250mL; 2.25g, Zhengzhou Yonghe Pharmaceutical Co., Ltd.
[0030] 4. 4-fluorouracil injection (5-Fu), specification 10mL: 0.25g / bottle, product of Shanghai Xudong Haipu Pharmaceutical Co., Ltd.:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com