Method to increase salt ion concentration of buffer to purify virus
A technology of salt ions and buffer solution, which is applied in the field of biopharmaceutical technology, can solve the problems of high cost and unsuitability for industrial production, and achieve the effects of reducing load, increasing load capacity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Amplification of Ad-HBx-hIL12 recombinant adenovirus
[0056] a) Expansion of cell seeds: take a cell from the HEK293 cell working bank stored in liquid nitrogen, and resuscitate to T175cm 2 square bottle, at 37°C, 5% CO 2 Cultivate in an incubator, and pass to the cell factory step by step after the cells are full.
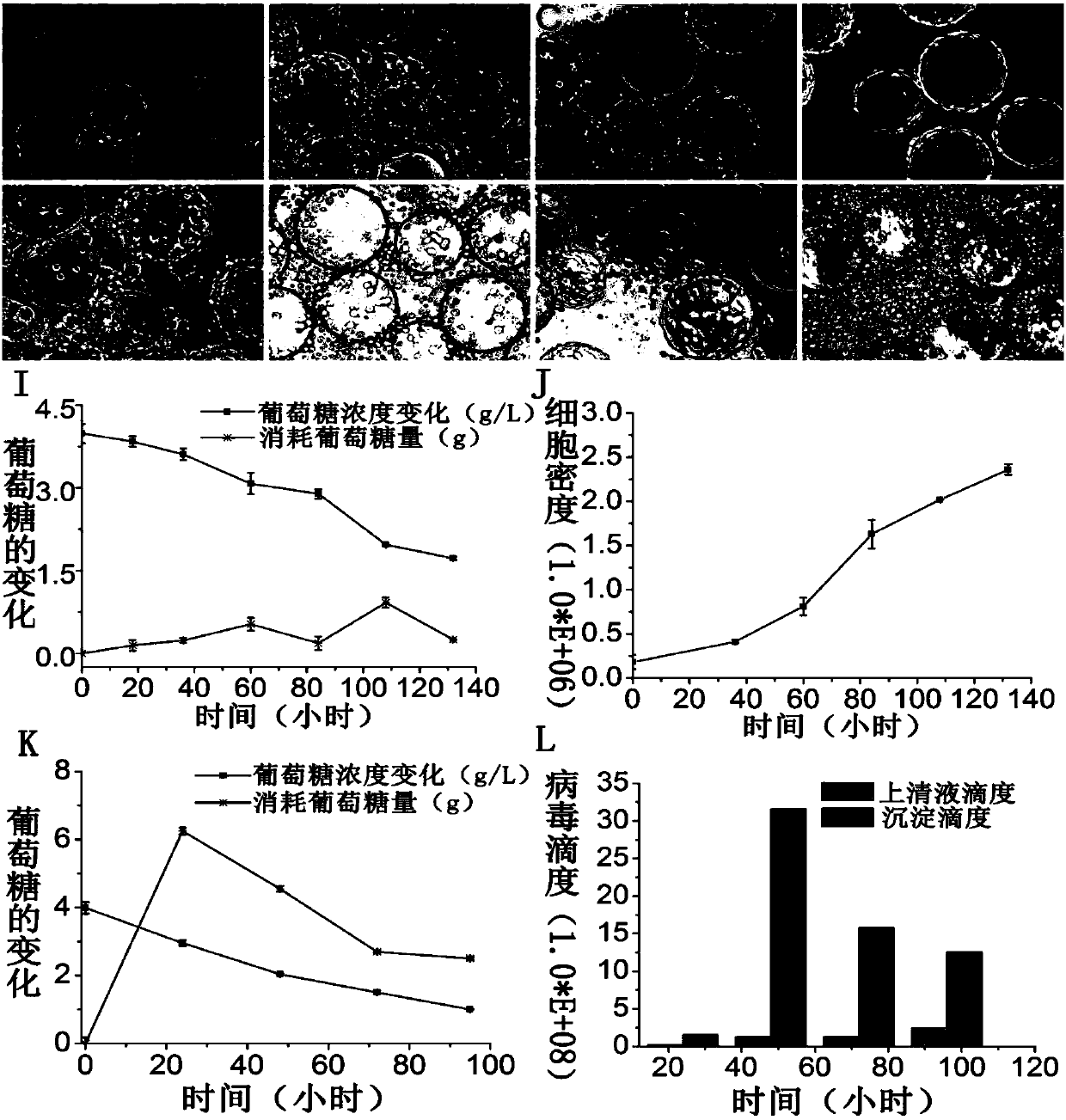
[0057] b) Expansion of cells in bioreactor: First, add cell culture medium into the bioreactor, when the operating conditions are stable at 37°C, pH 7.0, DO 50%, 50rpm, digest and collect HEK293 cells amplified by the cell factory, and inoculate in In the bioreactor, the inoculated cell density was 1.5×10 5 Cells / ml, add cell culture medium to 5L, the conditions of reactor cell culture are temperature 37℃, rotating speed 45rpm, pH 7.15-7.25, DO 30-50%, samples are taken every day to detect glucose concentration, cell density and micro Morphology of the cells on the vector.
[0058] c) Virus inoculation and virus amplification: when the cell d...
Embodiment 2
[0061] Example 2, Ultrafiltration Concentration and Filter Washing of Ad-HBx-hIL12 Recombinant Adenovirus
[0062] Take the virus supernatant collected in Example 1 at 6000rpm, centrifuge at a high speed at 4°C, discard the precipitate, keep the supernatant, and filter and clarify the centrifuged virus supernatant through a Burst filter; filter the clarified virus liquid with 300KD The membrane bag was concentrated, and the virus concentrate and filtrate were collected separately. Divide the concentrated sample into two parts, carry out 10 times volume filtration with low-salt filter buffer (100mM) and high-salt buffer (300mM) respectively, collect filter-wash samples and filter-wash samples respectively; Collect clarified Take 2ml of sample, concentrated sample, filtered sample, low-salt filtered sample, low-salt filtered sample, high-salt filtered sample, and high-salt filtered sample, add it to a glass tube, and observe the sample under natural light The change of phenol r...
Embodiment 3
[0064] Example 3 Optimum Filtration and Washing Volume of Recombinant Adenovirus Ad-HBx-hIL12
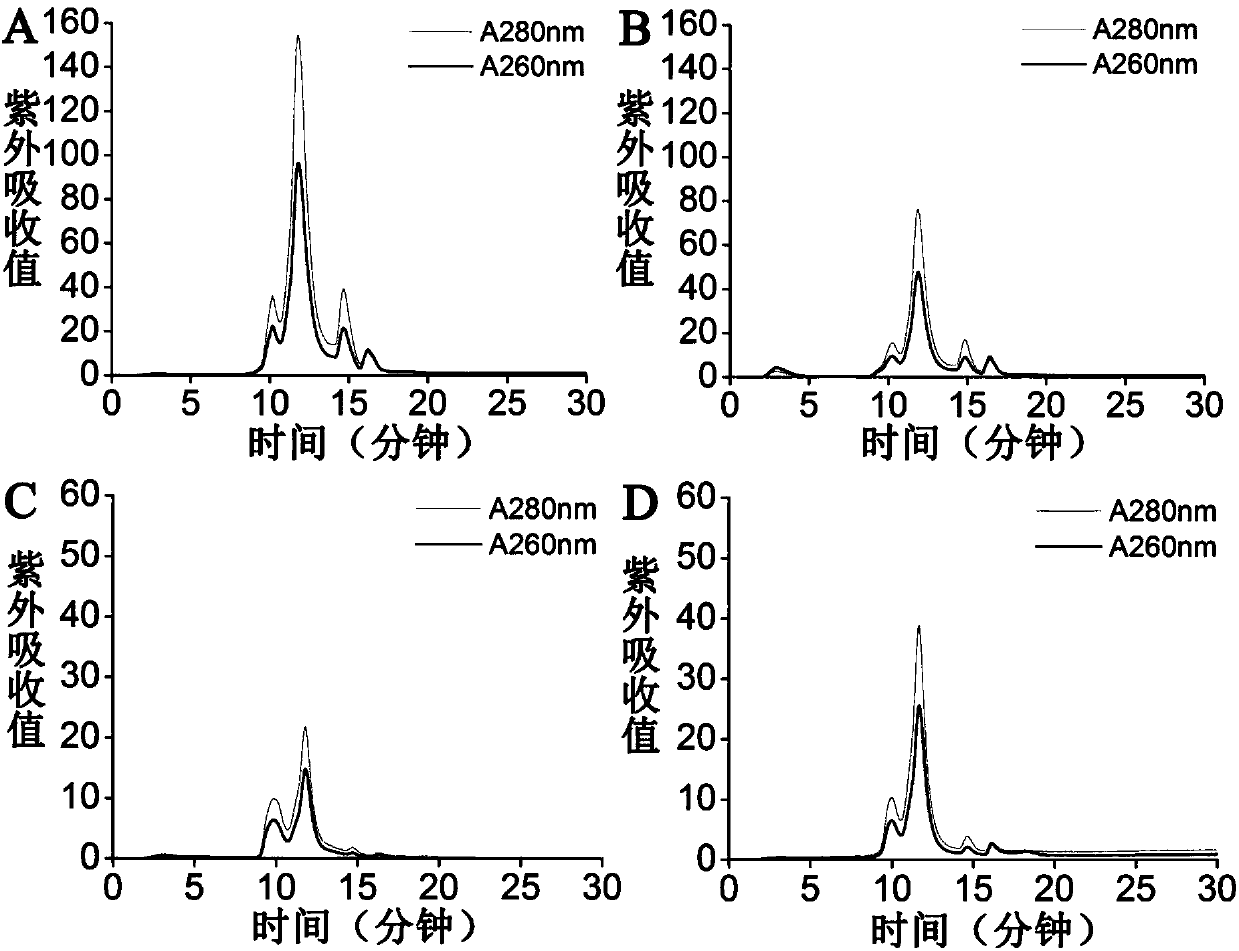
[0065]The steps of ultrafiltration concentration are the same as in Example 2, the difference being: the concentrated sample is subjected to 5 times, 10 times, 15 times, and 20 times conditional washing with high-salt filter washing buffer, and 5 times, 10 times, and 20 times are collected respectively. 15-fold and 20-fold filtered samples, 50mM Tris-HCl, 2mM MgCl 2 After dialysis with pH 8.0 buffer solution, 12.5% SDS-PAGE gel electrophoresis and Source15Q chromatographic analysis were used to detect the removal effect of impurity proteins; antibody staining and TCID50 method were used to detect the influence of filtration volume on virus activity,
[0066] Experimental results such as Figure 4 : With the increase of the volume of filtration and washing, the removal of impurity protein increases, and the activity of the virus will not change with the increase of the volume of f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com