Method for preparing the silicoaluminate form of the aei zeolite structure with high yields, and application in catalysis of method
A technology of zeolite and zeolite materials, applied to the high-yield preparation of aluminosilicates with AEI zeolite structure, can solve the problem of completely eliminating complexities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Synthesis of N,N-dimethyl-3,5-dimethylpiperidinium (DMDMP)
[0070] Mix 10 g of 3,5-dimethylpiperidine (Sigma-Aldrich, ≥96% by weight) with 19.51 g of potassium bicarbonate (KHCO 3 , Sigma-Aldrich; 99.7% by weight) were mixed and dissolved in 140 ml of methanol. Subsequently, 54ml methyl iodide (CH 3 I, Sigma-Aldrich, > 99% by weight), the resulting mixture was kept stirring at room temperature for 5 days. Once this time had elapsed, the reaction mixture was filtered to remove potassium bicarbonate. The filtered solution was partially concentrated by rotary evaporator. Once the methanol was partially evaporated, the solution was washed several times with chloroform and magnesium sulfate (MgSO 4 , Sigma-Aldrich, > 99.5% by weight). Subsequently, the mixture was filtered to remove magnesium sulfate. The ammonium salt was obtained by precipitation with diethyl ether followed by filtration. The final yield of N,N-dimethyl-3,5-dimethylpiperidinium iodide wa...
Embodiment 2
[0072] Example 2: Synthesis of an aluminosilicate form of the AEI zeolite structure using a zeolite with the FAU structure as the sole source of silicon and aluminum
[0073] 21.62 g of a 6.9% by weight aqueous solution of N,N-dimethyl-3,5-dimethylpiperidinium hydroxide were mixed with 1.89 g of a 20% by weight aqueous sodium hydroxide solution (NaOH, Sigma-Aldrich, 98%) mixed. The mixture was homogenized by maintaining stirring for 10 minutes. Finally, 3.01 g of zeolite (CBV-720, SiO 2 / Al 2 o 3 molar ratio = 21), and the mixture was kept stirring until the desired concentration was reached. The composition of the final gel is SiO 2 / 0.047Al 2 o 3 / 0.2DMDMP / 0.2NaOH / 15H 2 O. The gel was transferred to a Teflon-lined steel autoclave and heated at 135 °C under static conditions for 7 days. Once this time has elapsed, the resulting product is recovered by filtration, washed well with water and finally dried at 100°C. The material was calcined at 550° C. for 4 hours in ...
Embodiment 3
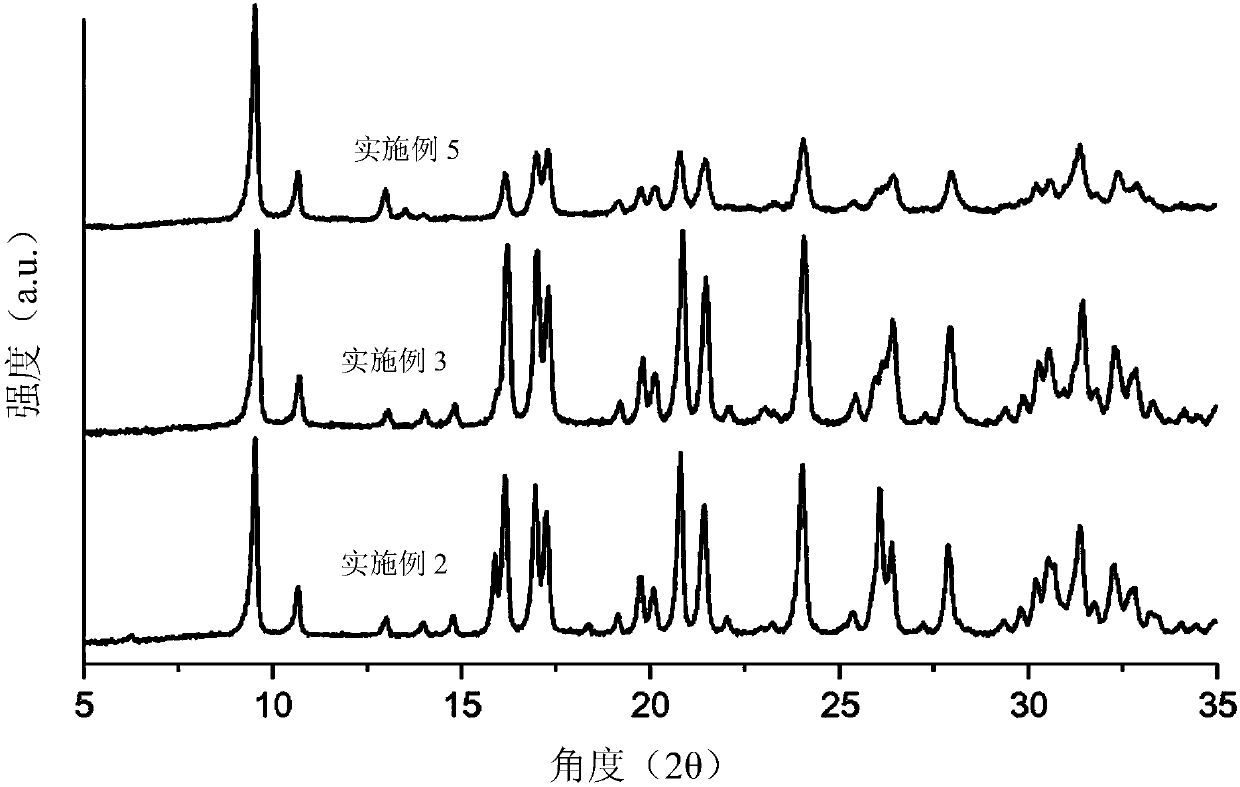
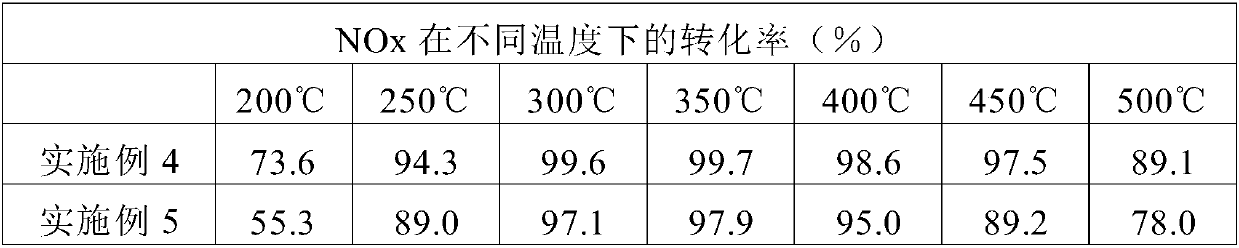
[0075] Example 3: Synthesis of an aluminosilicate form of the AEI zeolite structure using a zeolite with the FAU structure as the sole source of silicon and aluminum
[0076] 2.24 g of 7.4% by weight of N,N-dimethyl-3,5-dimethylpiperidine hydroxide in water and 0.173 g of 20% by weight of sodium hydroxide in water (NaOH, Sigma-Aldrich, 98 %)mix. The mixture was homogenized by maintaining stirring for 10 minutes. Finally, 0.193 g of zeolite (CBV-720, SiO 2 / Al 2 o 3 molar ratio = 21), and the mixture was kept stirring until the desired concentration was reached. The composition of the final gel is SiO 2 / 0.047Al 2 o 3 / 0.4DMDMP / 0.2NaOH / 15H 2 O. The gel was transferred to a Teflon-lined steel autoclave and heated at 135 °C under static conditions for 7 days. Once this period of time has elapsed, the resulting product is recovered by filtration, washed well with water and finally dried at 100°C. The material was calcined at 550° C. for 4 hours in an air atmosphere to e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com