Anticoagulant and antibacterial indwelling needle sleeve pipe and its preparation method
An indwelling needle and anti-coagulation technology, which is applied in the field of medical devices, can solve the problems of less exploration of antibacterial function of the coating, great influence on the environment, and weak bonding strength, etc., and achieve long-lasting anti-coagulation and antibacterial effects and a wide range of sterilization , High adhesion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
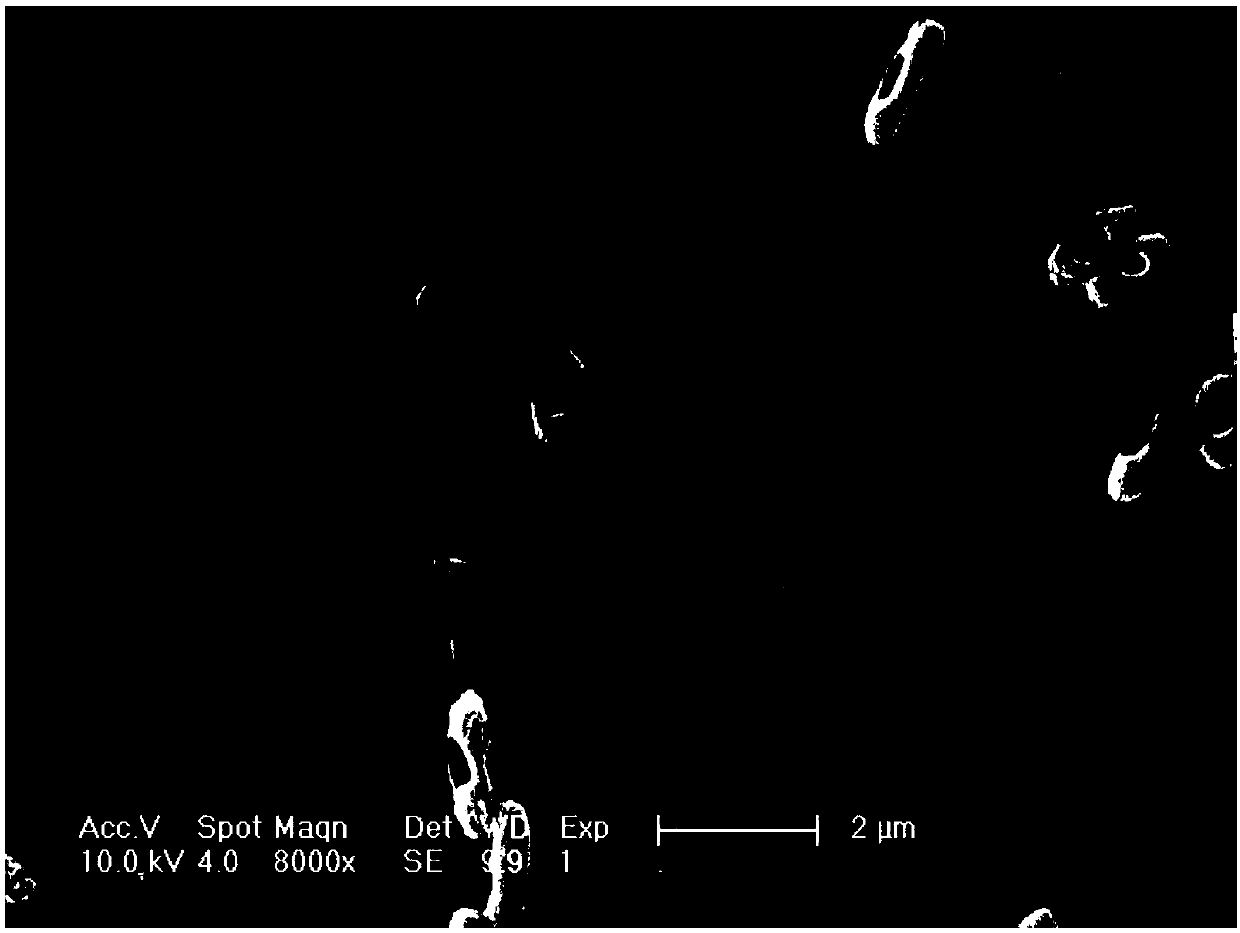
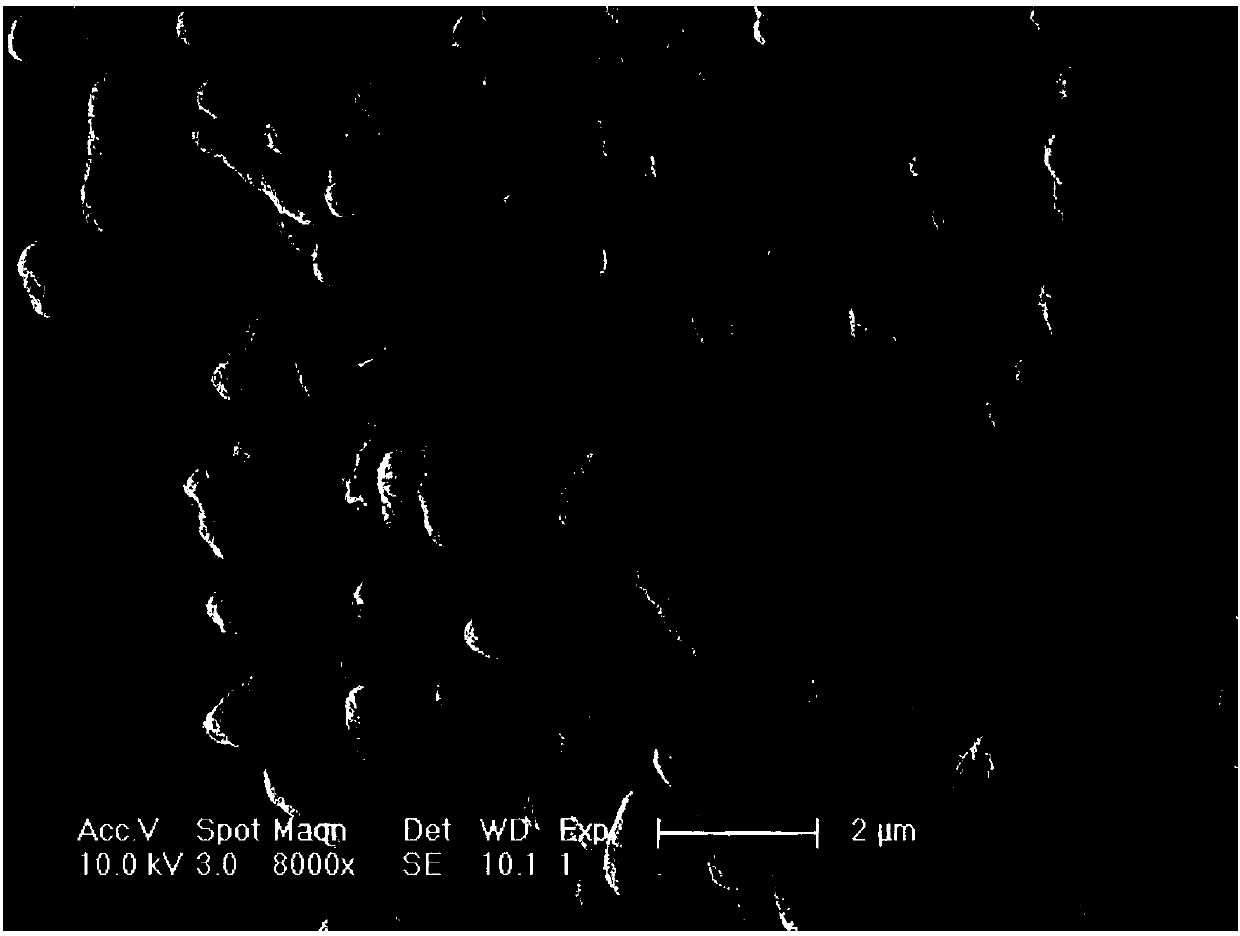
Image
Examples
preparation example Construction
[0082] The present invention provides a method for preparing an anticoagulant and antibacterial indwelling needle sleeve described in the above technical solution, comprising the following steps:
[0083] A) reacting the hydrogen-absorbing quaternary ammonium salt solution with the structure of formula I and the heparin sodium solution to obtain the hydrogen-absorbing quaternary ammonium salt heparin complex;
[0084]
[0085] In formula I, R is selected from
[0086] R 1 and R 2 Alkyl independently selected from H or C1~C4; R 3 Alkyl group selected from C8~C16;
[0087] x - from Cl - 、Br - or I - ;
[0088] B) dissolving the hydrogen-absorbing quaternary ammonium salt heparin complex obtained in the step A) in an organic solvent, and then loading the obtained complex solution on the indwelling needle sleeve;
[0089] C) UV-curing the indwelling needle sleeve attached with the compound obtained in the step B) to obtain an anticoagulant and antibacterial indwellin...
Embodiment 1
[0104] Preparation of anticoagulant and antibacterial indwelling needle sleeve
[0105] A) Dissolve N-(4-benzoylbenzyl)-N,N-dimethyldodecyl-1-ammonium bromide in ultrapure water and prepare a hydrogen pumping solution with a concentration of 0.1g / ml Type quaternary ammonium salt solution; dissolve heparin sodium (molecular weight 7500-20000g / mol) in ultrapure water to prepare a heparin sodium solution with a concentration of 0.1g / ml; add the above-mentioned hydrogen pumping type quaternary ammonium salt solution drop by drop into 5ml of heparin sodium solution until a large amount of white precipitate precipitates out of the solution, and after standing for 30 minutes, filter under reduced pressure to obtain a white precipitate. The precipitate was washed three times with ultrapure water, and freeze-dried to obtain the hydrogen-absorbing quaternary ammonium salt heparin complex.
[0106] B) Dissolve the hydrogen-absorbing quaternary ammonium heparin complex obtained in the st...
Embodiment 2
[0109] A) Dissolve 4-(4-(diethylamino)benzoyl)-N,N-diethyl-N-octylphenylammonium iodide in ultrapure water to prepare a concentration of 0.125g / ml of hydrogen pumping type quaternary ammonium salt solution; dissolve heparin sodium (molecular weight 7500~20000g / mol) in ultrapure water to prepare a heparin sodium solution with a concentration of 0.125g / ml; the above hydrogen pumping type quaternary ammonium salt solution Add dropwise to 5ml of heparin sodium solution until a large amount of white precipitate precipitates out of the solution. After standing still for 30 min, the white precipitate was obtained by filtration under reduced pressure. The precipitate was washed three times with ultrapure water, and freeze-dried to obtain the hydrogen-absorbing quaternary ammonium salt heparin complex.
[0110] B) Dissolve the hydrogen-absorbing quaternary ammonium heparin complex obtained in the step A) in chloroform, and the prepared concentration is a 2% hydrogen-absorbing quatern...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| survival rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com