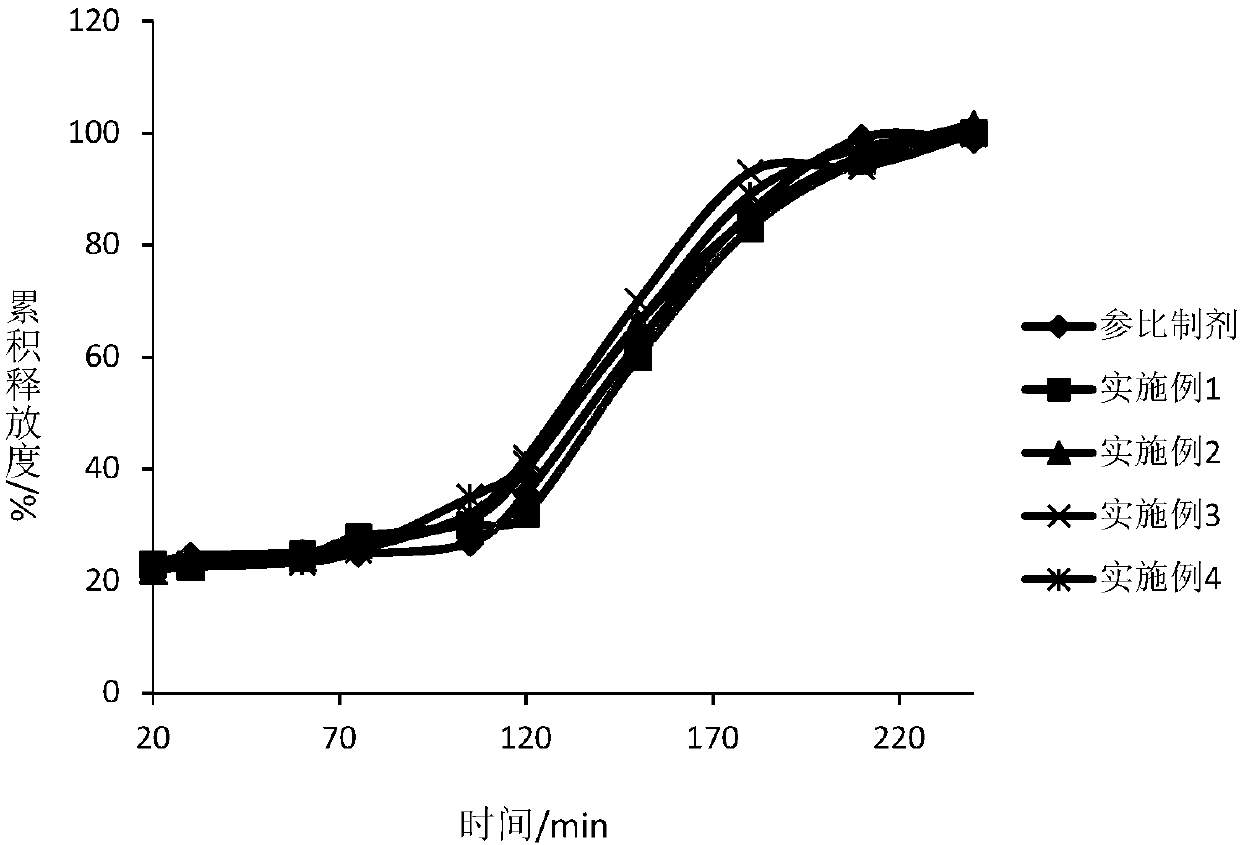
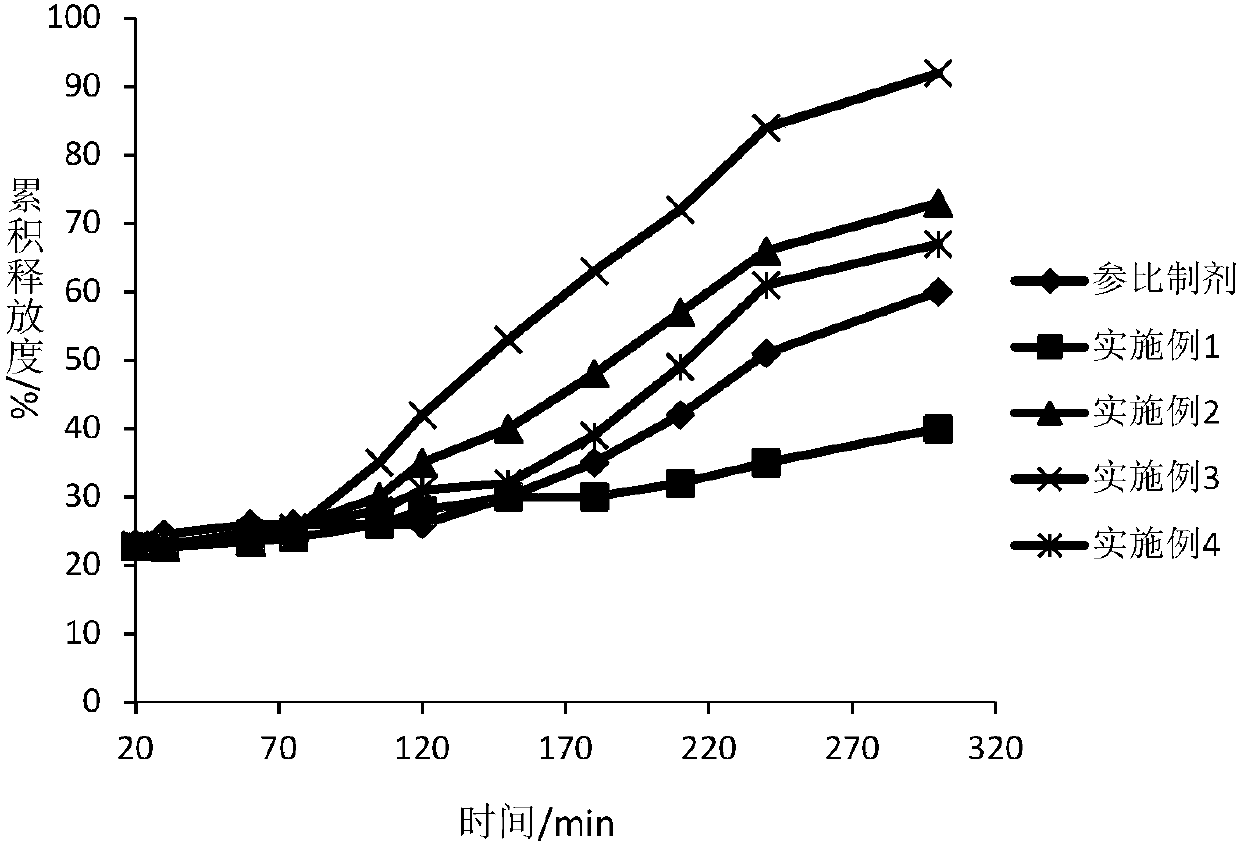
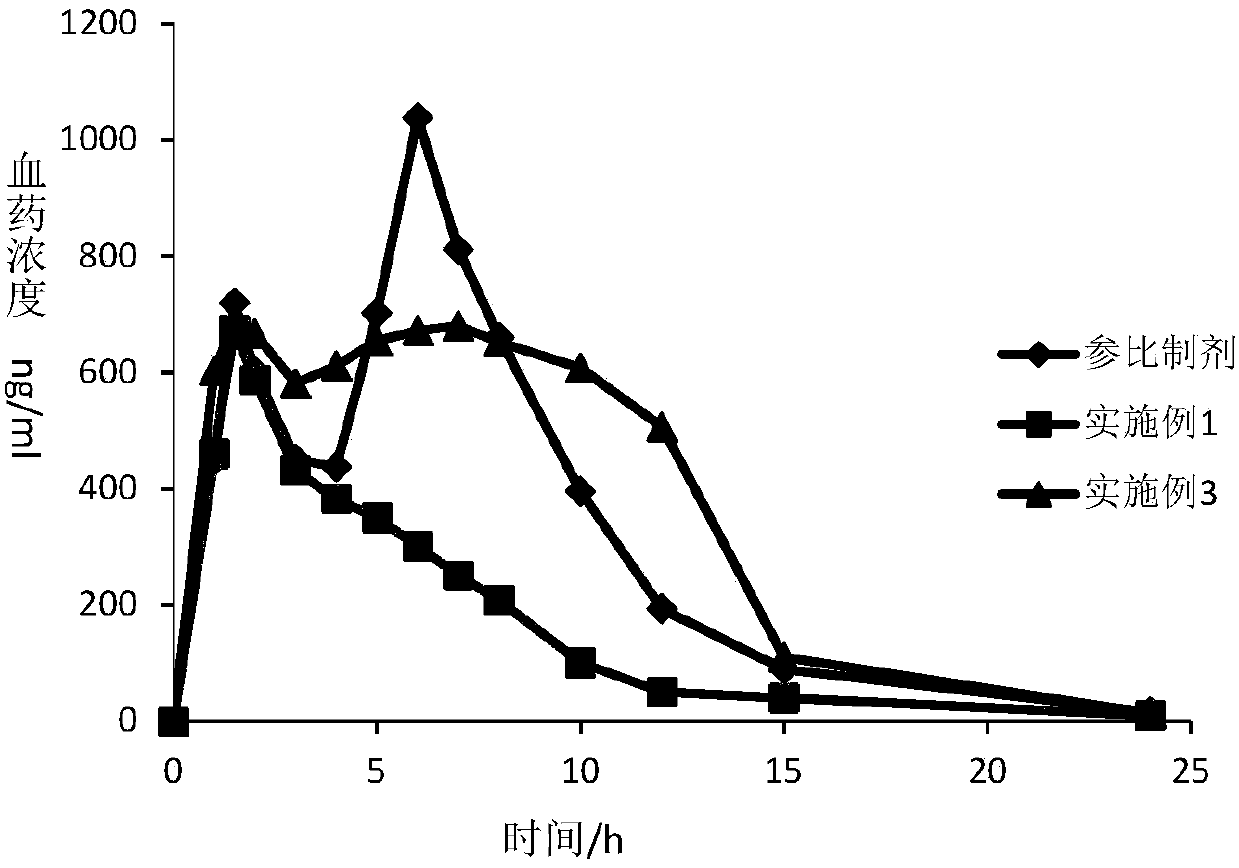
Dexlansoprazole medicinal preparation
A dexlansoprazole and drug technology, applied in the field of pharmaceutical preparations, can solve the problems of inappropriate proportion, non-absorption, poor acid control effect, etc., and achieve the effects of stable drug release, long maintenance time, and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: A kind of dexlansoprazole delayed capsule composition and preparation method thereof
[0088] Enteric-coated immediate-release pellets:
[0089]
[0090] Delayed pellet prescription composition:
[0091]
[0092] The preparation process of enteric-coated quick-release pellets: (1) Weigh the prescription amount of dexlansoprazole, sucrose, heavy magnesium carbonate, and hypromellose, mix them evenly, place them in the feed funnel, and place the medicinal sucrose pellet cores in the In a coating granulator, 2% (w / w) hydroxypropyl cellulose aqueous solution is used as a binder, and the drug-containing pellets are prepared by centrifugal granulation. Dry the drug-containing pellets at 35°C±5°C until the water content is less than 5%, and sieve the pellets between 18 mesh and 24 mesh for use.
[0093] (2) Place the drug-containing pellets in a fluidized bed, preheat to the material temperature, adopt the Hüttlin bottom spray fluidized bed coating techno...
Embodiment 2
[0098] Embodiment 2: A kind of dexlansoprazole delayed capsule composition and preparation method thereof
[0099] Enteric-coated immediate-release pellets:
[0100]
[0101]
[0102] Delayed pellet prescription composition:
[0103]
[0104] The preparation process of enteric-coated quick-release pellets: (1) Weigh the prescription amount of dexlansoprazole, sucrose, heavy magnesium carbonate, and hypromellose, mix them evenly, place them in the feed funnel, and place the medicinal sucrose pellet cores in the In a coating granulator, 2% (w / w) hydroxypropyl cellulose aqueous solution is used as a binder, and the drug-containing pellets are prepared by centrifugal granulation. Dry the drug-containing pellets at 35°C±5°C until the water content is less than 5%, and sieve the pellets between 18 mesh and 24 mesh for use.
[0105] (2) Place the drug-containing pellets in a fluidized bed, preheat to the material temperature, adopt the Hüttlin bottom spray fluidized bed co...
Embodiment 3
[0110] Embodiment 3: A kind of dexlansoprazole delayed capsule composition and preparation method thereof
[0111] Enteric-coated immediate-release pellets:
[0112]
[0113] Delayed pellet prescription composition:
[0114]
[0115]
[0116] The preparation process of enteric-coated quick-release pellets: (1) Weigh the prescription amount of dexlansoprazole, sucrose, heavy magnesium carbonate, and hypromellose, mix them evenly, place them in the feed funnel, and place the medicinal sucrose pellet cores in the In a coating granulator, 2% (w / w) hydroxypropyl cellulose aqueous solution is used as a binder, and the drug-containing pellets are prepared by centrifugal granulation. Dry the drug-containing pellets at 35°C±5°C until the water content is less than 5%, and sieve the pellets between 18 mesh and 24 mesh for use.
[0117] (2) Place the drug-containing pellets in a fluidized bed, preheat to the material temperature, adopt the Hüttlin bottom spray fluidized bed coat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com