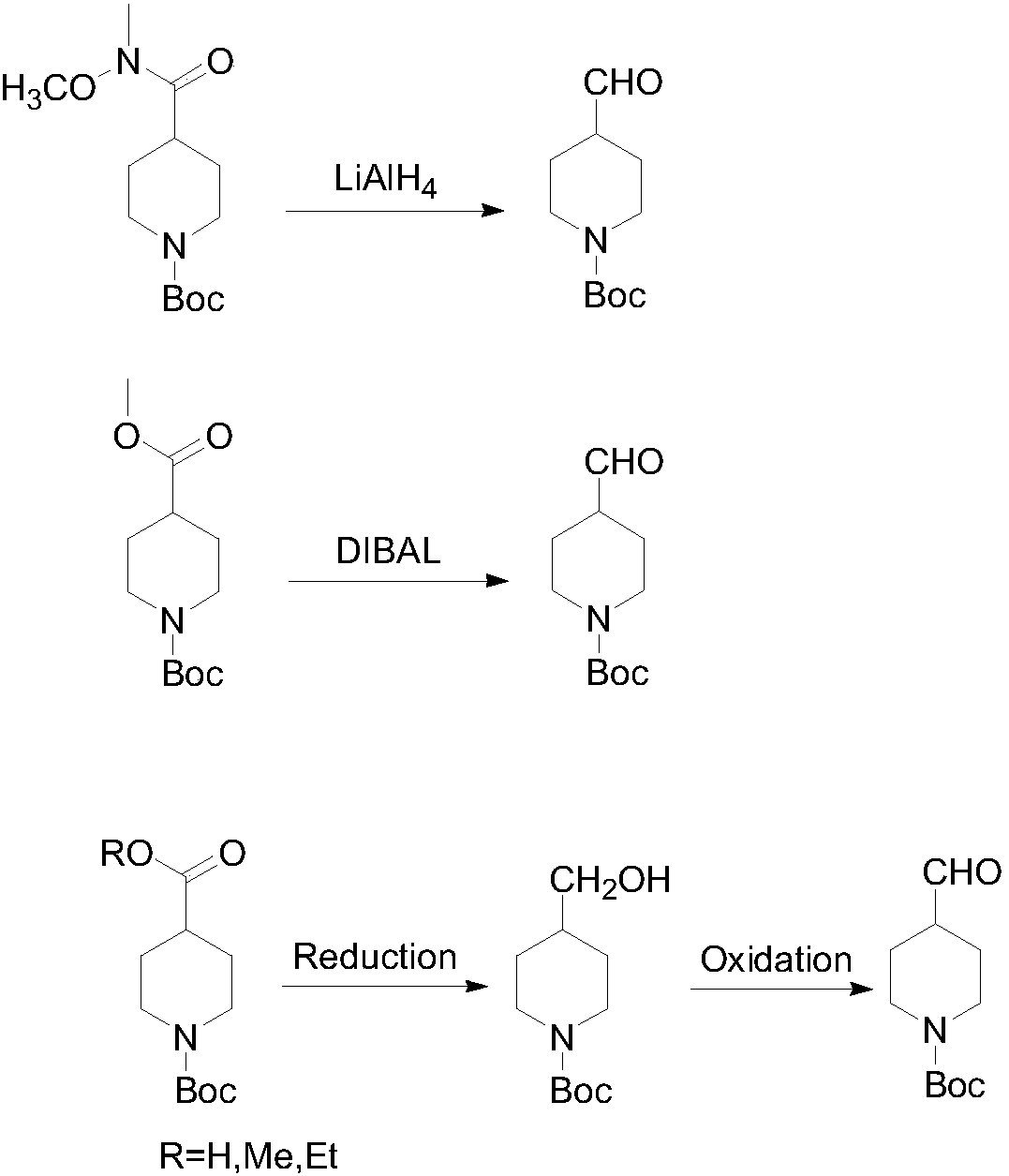
Preparing method of N-Boc-4-piperidine arboxyaldehyde
A technology of piperidine formaldehyde and n-boc-4-, which is applied in the field of preparation of N-Boc-4-piperidine formaldehyde, can solve the problems of easy safety accidents, low product purity, and easy generation of impurities, so as to avoid excessive Effects of oxidation or reduction, high yield and purity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
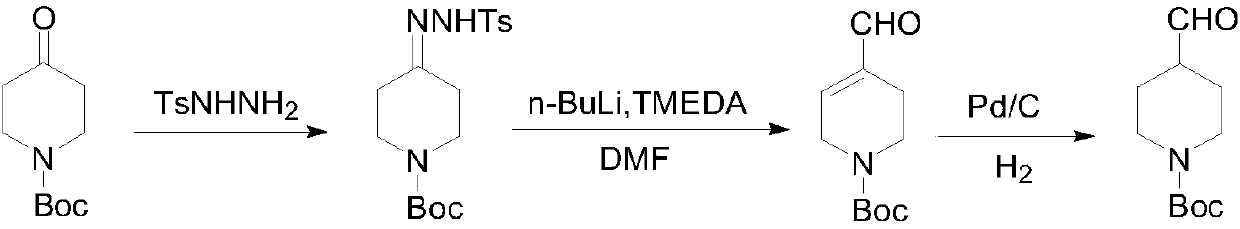
Embodiment 1
[0030] 1. Add 18.62g of p-toluenesulfonylhydrazide (0.10mol, 1.0eq), 19.92g of N-Boc-4-piperidone (0.10mol, 1.0eq), 100mL of methanol to a 250mL single-necked bottle with magnetic stirring, and heat to reflux After 3 hours, it was cooled to room temperature, filtered, and dried to obtain 32.78 g of p-toluenesulfonyl ketone hydrazone as a white solid, with an HPLC content of 99.0% and a yield of 89.23%.
[0031] 2. Under the protection of nitrogen, the system was cooled to -70°C with a dry ice / ethanol external bath, and 178mL of n-butyl lithium in n-hexane solution (2.5mol / L, 0.445mol, 5eq ) and 95mL tetrahydrofuran were added dropwise to a solution of 32.78g p-toluenesulfonyl ketone hydrazone (0.089mol, 1eq) dissolved in 51.71g tetramethylethylenediamine (0.445mol, 5eq) in the dropping funnel. Keep warm for 3 hours. Continue to lower the temperature to below -70°C, add 13.01g DMF (0.178mol, 2.0eq) dropwise, after dropping, keep warm for 1 hour, naturally rise to room temperat...
Embodiment 2
[0035] 1. Add 18.62g of p-toluenesulfonylhydrazide (0.10mol, 1.0eq), 19.92g of N-Boc-4-piperidone (0.10mol, 1.0eq), 100mL of ethanol to a 250mL single-necked bottle with magnetic stirring, and heat to reflux After 3 hours, it was cooled to room temperature, filtered, and dried to obtain 33.42 g of p-toluenesulfonyl ketone hydrazone as a white solid, with an HPLC content of 98.5% and a yield of 90.96%.
[0036] 2. Under the protection of nitrogen, the system was cooled to -70°C with a dry ice / ethanol external bath, and 146mL of n-butyl lithium in n-hexane (2.5mol / L, 0.364mol, 4eq ) and 65mL of tetrahydrofuran were added dropwise to a solution of 33.42g p-toluenesulfonyl ketone hydrazone (0.091mol, 1eq) dissolved in 42.28g tetramethylethylenediamine (0.364mol, 4eq) in the dropping funnel. Keep warm for 3 hours. Continue to cool down to below -70°C, add 20.60g of N-formylpiperidine (0.182mol, 2.0eq) dropwise, after dropping, keep warm for 1 hour, naturally rise to room temperatu...
Embodiment 3
[0039] 1. Add 35.38g of p-toluenesulfonylhydrazide (0.19mol, 0.95eq), 39.85g of N-Boc-4-piperidone (0.20mol, 1.0eq), 100mL of isopropanol to a 500mL single-necked bottle with magnetic stirring, Heated to reflux for 3 hours, lowered to room temperature, filtered, and dried to obtain 65.78 g of p-toluenesulfonyl ketone hydrazone as a white solid, with an HPLC content of 98.3% and a yield of 89.52%.
[0040] 2. Under the protection of nitrogen, the system was cooled to -40°C with a dry ice / ethanol external bath, and 358mL of n-butyl lithium in n-hexane (2.5mol / L, 0.896mol, 5eq ) and 80mL tetrahydrofuran, add dropwise the solution of 65.78g p-toluenesulfonyl ketone hydrazone (0.179mol, 1eq) dissolved in 104.06g tetramethylethylenediamine (0.896mol, 5eq) in the dropping funnel, after dropping, naturally rise to room temperature Keep warm for 3 hours. Continue to cool down to below -40°C, add 19.62g DMF (0.268mol, 1.5eq) dropwise, after dropping, keep warm for 1 hour, naturally ris...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com