Preparation method of vanadium trioxide
A technology of vanadium trioxide and ammonium vanadate, which is applied to vanadium oxide, electrochemical generators, fuel cells, etc., can solve the problems of complicated operation and high production process cost, and achieve simple production process operation, reduced production cost, and Coulomb Efficiency and energy efficiency without attenuation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
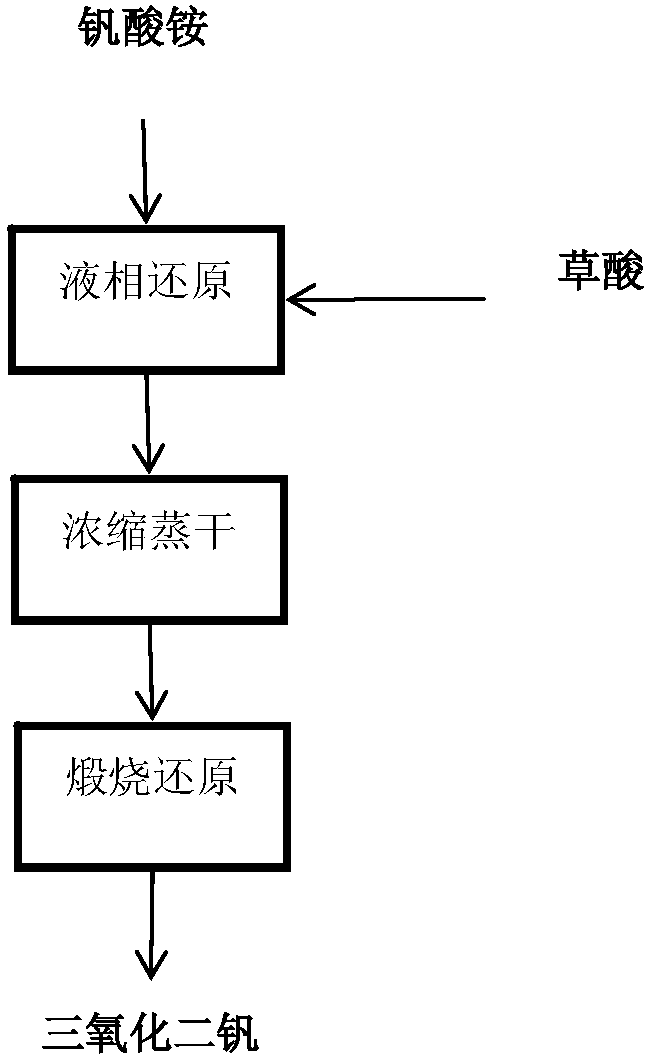
[0040] The invention provides a preparation method of vanadium trioxide, comprising the following steps (see the production process schematic diagram figure 1 ):
[0041] 1) Mix ammonium metavanadate or ammonium polyvanadate with oxalic acid (the mixing ratio is, the molar amount of vanadium: the molar amount of oxalic acid = 1:1.5~1:2.5), and add it to the deionized In water (the molar amount of vanadium: the volume of water is 8.5-10mol / L), carry out liquid phase reduction;
[0042] 2) After ammonium metavanadate or ammonium polyvanadate is completely dissolved, heat to boiling, concentrate the solution and evaporate to dryness to obtain a precursor;
[0043] 3) Calcining the obtained precursor for 2-4 hours under the protection of nitrogen at a temperature of 700-900° C., and cooling to room temperature under the protection of nitrogen to obtain vanadium trioxide.
[0044] In this production process, the main component obtained is (NH 4 ) 2 (VO) 2 (C 2 o 4 ) 3 The p...
Embodiment 1
[0047] Embodiment 1 adopts technique of the present invention to prepare vanadium trioxide
[0048]1) Mix analytically pure ammonium metavanadate with analytically pure oxalic acid dihydrate (mixing ratio: molar mass of vanadium: molar mass of oxalic acid = 1:1.5), and add in batches to deionized water at a temperature of 80°C (vanadium The molar weight: the volume of water=10mol / L), carry out liquid phase reduction;
[0049] 2) After the ammonium metavanadate is completely dissolved, heat to boiling, concentrate the solution and evaporate to dryness to obtain the precursor;
[0050] 3) After the obtained precursor was dried at 160°C, it was calcined for 2.5h under the protection of nitrogen at a temperature of 700°C, and cooled to room temperature under the protection of nitrogen. Finally, the purity of the obtained vanadium trioxide is 98.8%.
Embodiment 2
[0051] Embodiment 2 adopts technique of the present invention to prepare vanadium trioxide
[0052] 1) Mix analytically pure ammonium metavanadate with analytically pure oxalic acid dihydrate (mixing ratio: molar mass of vanadium: molar mass of oxalic acid = 1:2), and add in batches to deionized water at a temperature of 95°C (vanadium The molar weight: the volume of water=9mol / L), carry out liquid phase reduction;
[0053] 2) After the ammonium metavanadate is completely dissolved, heat to boiling, concentrate the solution and evaporate to dryness to obtain the precursor;
[0054] 3) The obtained precursor was dried at 100° C., calcined for 3 h under the protection of nitrogen at a temperature of 750° C., and cooled to room temperature under the protection of nitrogen. Finally, the purity of the obtained vanadium trioxide is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com