HPLC analysis method for (S)-2-aminopropanol
An aminopropanol and analysis method technology, applied in the field of high-performance liquid chromatography analysis of the chiral purity of -2-aminopropanol, can solve problems affecting levofloxacin, chiral isomers with weak ultraviolet absorption, etc., and facilitate standardized operation , stable properties and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: The chiral purity analysis of (RS)-2-aminopropanol
[0026] Precisely weigh about 25mg of (RS)-2-aminopropanol and put it in a 25ml measuring bottle, add about 0.2ml of derivatization reagent (derivatization reagent: 18g of tetrafluorobenzoic acid acetic anhydride, diluted with 200ml of anhydrous methanol), add mobile phase to dilute To the mark, shake well, take 0.5ml and add mobile phase to dilute to 10ml, as the test solution.
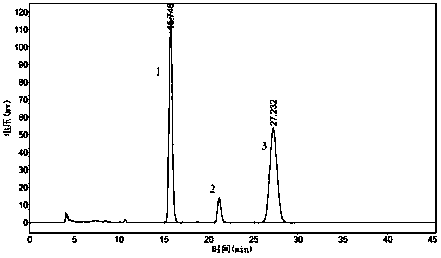
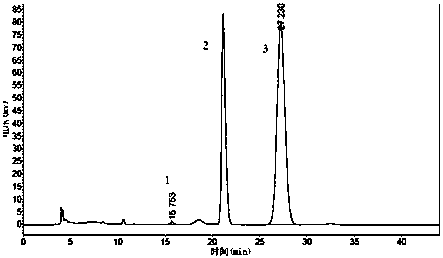
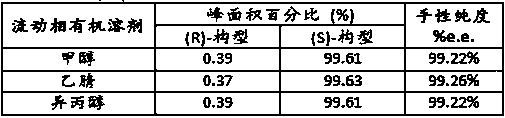
[0027] (1) Liquid chromatography condition A: ChromTech CHIRAL-AGP column, the mobile phase is 0.015mol / L phosphate aqueous solution-isopropanol (99:1), the UV detection wavelength is 320nm, the flow rate is 0.8 mL / min, the column The temperature was 30°C, and the injection volume was 20 μL. Retention time, resolution and chiral purity are shown in Table 1 below, and the spectrum is shown in figure 1 .
[0028] (2) Liquid chromatography condition B: ChromTech CHIRAL-AGP column, the mobile phase is 0.015 mol / L phosphate aqueou...
Embodiment 2
[0032] Embodiment 2: The chiral purity analysis of (S)-2-aminopropanol
[0033] Precisely weigh about 25 mg of (S)-2-aminopropanol and put it in a 25 ml measuring bottle, add about 0.2 ml of derivatization reagent (derivatization reagent: 18 g of tetrafluorobenzoic acid acetic anhydride, diluted with 200 ml of anhydrous methanol), add mobile phase to dilute To the mark, shake well, take 0.5ml and add mobile phase to dilute to 10ml, as the test solution.
[0034] (1) Liquid chromatography condition A: ChromTech CHIRAL-AGP column, the mobile phase is 0.015mol / L phosphate aqueous solution-isopropanol (99:1), the UV detection wavelength is 320nm, the flow rate is 0.8 mL / min, the column The temperature was 30°C, and the injection volume was 20 μL. Retention time, resolution and chiral purity are shown in Table 2 below, and the spectrum is shown in figure 2 .
[0035] (2) Liquid chromatography condition B: ChromTech CHIRAL-AGP column, the mobile phase is 0.015 mol / L phosphate aq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com