Lyophilized preparation and preparation method thereof
A freeze-dried preparation and drying technology, applied in the field of freeze-dried preparations and their preparation, can solve the problems of single preparation shape, large adhesive force, single preparation components, etc., and achieve good preparation strength, fast disintegration speed, and good solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The invention provides a preparation method of a freeze-dried preparation, comprising the following steps:
[0061] a) mixing and freezing the preparation raw materials to obtain a soft ice mixture, the preparation raw materials include active ingredients, a binder and water; the mass ratio of the active ingredients to the binder is 0-100:0-100, and they are The content is not 0 at the same time;
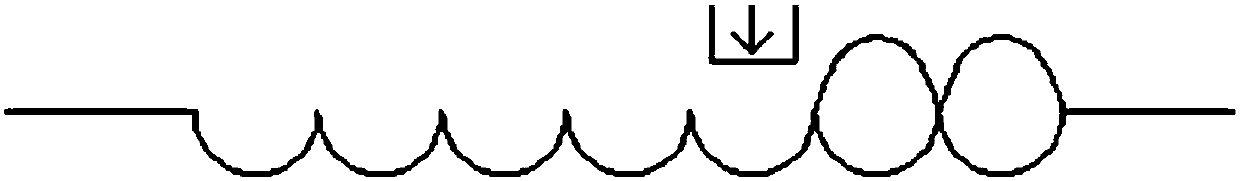
[0062] b) using a filling machine to release the soft ice mixture into the environment to solidify and form, and the filling machine is selected from a pipetting device, a plunger pump, a screw pump, a gear pump or a peristaltic pump to obtain a solidified product;
[0063] c) Freeze-drying the solidified product to obtain a freeze-dried preparation.
[0064] The freeze-dried preparation prepared by the preparation method provided by the invention has a controllable shape and a high drug loading amount. In addition, the freeze-dried preparation prepared by the method has go...
Embodiment 1
[0144] 35g of vitamin C, 10g of pullulan, 25g of mannitol, and 1 liter of water were prepared into a mixed solution to form Liquid 1;
[0145] Freeze liquid 1 at -11°C, and at the same time inflate to an expansion rate of 115% to form a soft ice mixture, which is component 2;
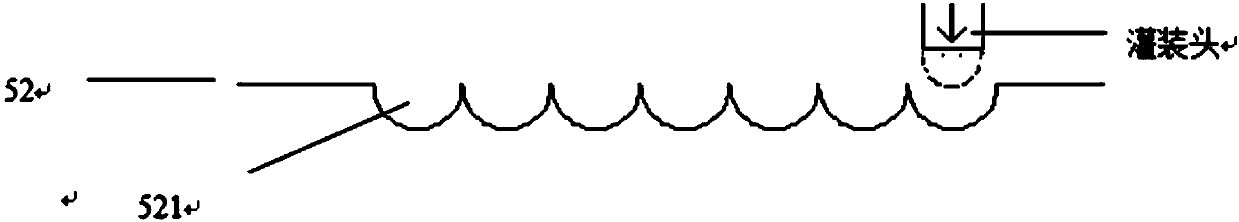
[0146] The surface of the oval-shaped concave die 51 and the flat die 52 combined mold at -150 ° C; Docking with the die 521 in turn to form a complete shape with a high degree of close contact; freeze component 2 to a certain amount to form component 6;
[0147] The quantitative component 6 and the flat mold 52 are freeze-dried together to form a freeze-dried oral VC health food, namely a freeze-dried preparation.
[0148] In this example, the strength, disintegration rate and solubility of the freeze-dried preparation were tested according to the test method described in the above technical solution, and the test results were: the preparation strength was good (friability <0.1%), the disintegration r...
Embodiment 2
[0150] 2 g of guar gum, 8 g of glycerin, 35 g of mannitol, and 1 liter of water were prepared into a mixed solution to form Liquid 1;
[0151] Freeze liquid 1 at -8°C, and at the same time inflate to an expansion rate of 135% to form a soft ice mixture, which is component 2;
[0152] Add vitamin C 55g in the form of dry powder to component 2, with uniform stirring, to form a soft ice mixture, which is component 4;
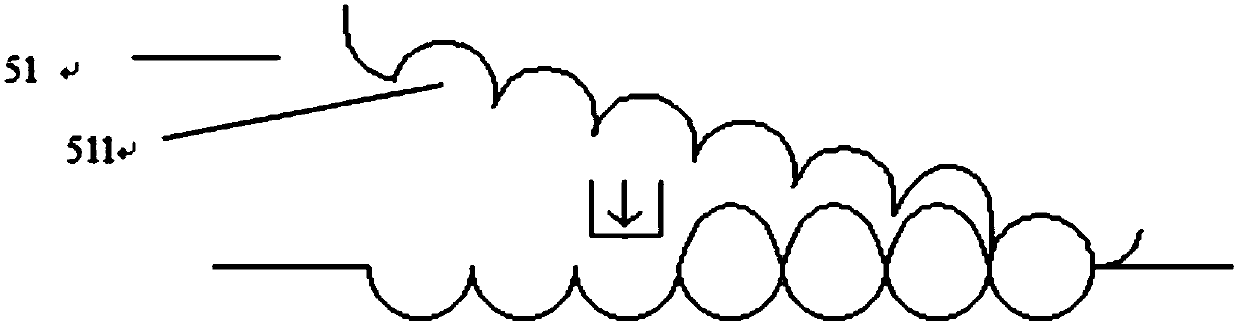
[0153] Combine the rolling die 51 with the spindle-shaped concave die on the surface and the flat die 52 to pre-cool at -86°C; fill the component 4 into the concave die 521, rotate the rolling die 51, and move the flat die 52 in parallel to make the concave die 511 Docking with the die 521 in turn to form a complete form with a high degree of close contact; freeze component 4 to a certain amount to form component 6;
[0154] Continue to reduce the temperature of quantitative component 6 to -145°C, and release from the mold;
[0155] The flat mold is turned over t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com