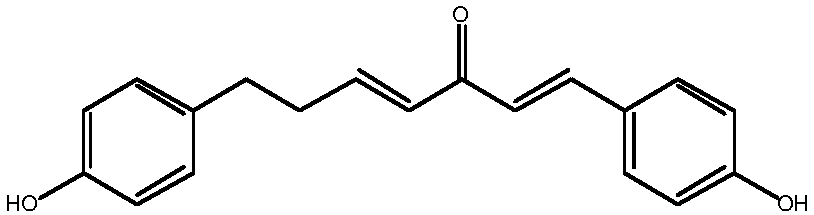
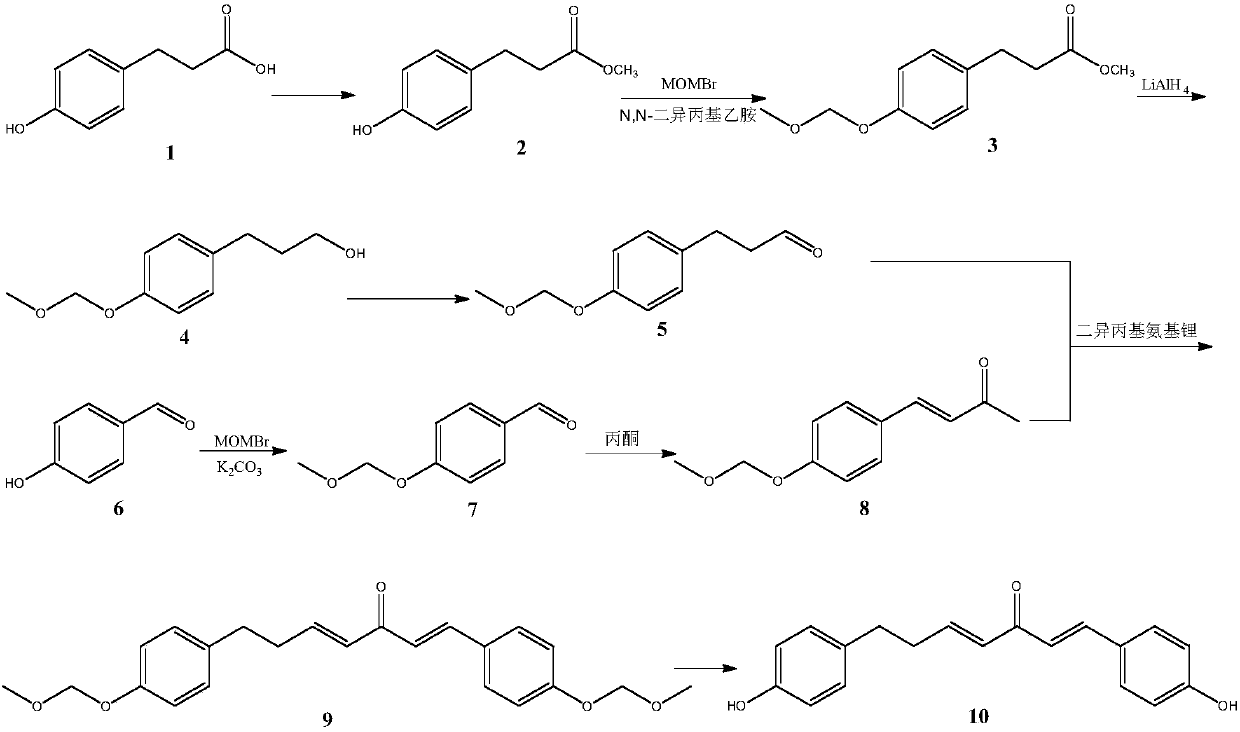
Method for synthesizing 1, 7-2-(4-hydroxy phenyl)-heptane-1, 4-diene-3-ketone
A technology of hydroxyphenyl and synthetic method, which is applied in the direction of chemical instruments and methods, preparation of organic compounds, drug combination, etc., can solve the problems of high price, low purity, and low content, and achieve simple method, high purity, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Preparation of 3-(4-hydroxyphenyl) methyl propionate
[0046] 3-(4-Hydroxyphenyl)propanoic acid (8g, 48.14mmol) was dissolved in methanol (40mL), and concentrated sulfuric acid (0.4mL) was added dropwise to catalyze the reaction at 35°C for 4h, and part of the methanol was removed by concentration under reduced pressure. Add 10 mL of water, adjust the pH to 6 with saturated sodium bicarbonate, extract three times with 20 mL of ethyl acetate, wash three times with 20 mL of saturated brine, dry over anhydrous sodium sulfate, and remove ethyl acetate under reduced pressure to obtain 3-(4- hydroxyphenyl) propionate methyl ester.
[0047] 2. Preparation of methyl 3-(4-(methoxymethyl)phenyl)propionate methyl ester
[0048]Methyl 3-(4-hydroxyphenyl)propionate (3 g, 16.66 mmol) and N,N-diisopropylethylamine (3.5 mL, 21.65 mmol) were dissolved in anhydrous dichloromethane (50 mL) with stirring , stirred for 30min, added acetone 50mL, added dropwise bromomethyl methyl ether ...
Embodiment 2
[0061] 1. Prepare 3-(4-hydroxyphenyl) ethyl propionate
[0062] 3-(4-Hydroxyphenyl)propanoic acid (8g, 48.14mmol) was dissolved in ethanol (40mL), concentrated sulfuric acid (0.4mL) was added dropwise to catalyze the reaction at 35°C for 4h, and part of the methanol was removed by concentration under reduced pressure. Add 10 mL of water, adjust the pH to 6 with saturated sodium bicarbonate, extract three times with 20 mL of ethyl acetate, wash three times with 20 mL of saturated brine, dry over anhydrous sodium sulfate, and remove ethyl acetate under reduced pressure to obtain 3-(4- hydroxyphenyl) ethyl propionate.
[0063] 2. Preparation of ethyl methyl 3-(4-(methoxymethyl)phenyl)propionate
[0064] Ethyl 3-(4-hydroxyphenyl)propionate (3 g, 16.66 mmol) and N,N-diisopropylethylamine (3.5 mL, 21.65 mmol) were dissolved in anhydrous dichloromethane (50 mL) with stirring , stirred for 30min, added acetone 50mL, added dropwise bromomethyl methyl ether (1.7mL, 21.65mmol), heated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com