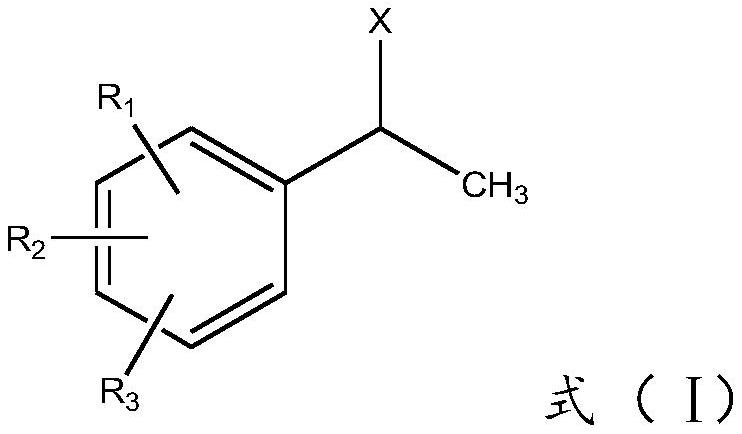
A kind of preparation method of 1-arylethanesulfonic acid and derivative thereof
A technology of arylethanesulfonic acid and arylethanethiol, which is applied in the field of preparation of 1-arylethanesulfonic acid and derivatives thereof, can solve the problems of difficult separation, increased equipment cost, low reaction yield and the like, and achieves Mild reaction conditions, reduced environmental pressure, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
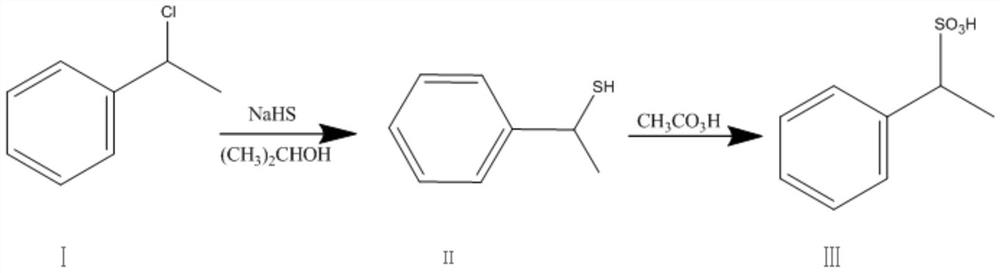
[0033] In a 1000ml four-necked bottle equipped with a stirrer, a condenser, and a thermometer, add 100ml of isopropanol and 20.3g of sodium hydrosulfide, start stirring, and start to add 34.0g of compound I dropwise, at a temperature of 30-35°C, dropwise 1-1.5 hours, after the dropwise addition, the temperature is raised to 50°C for heat preservation reaction for 10 hours, the heat preservation temperature is 50-55°C, controlled by GC, after the reaction is completed, the reaction is concentrated under reduced pressure, the organic solvent is recovered and the reaction is applied, and the obtained oil is Compound II . Then, 300.0 g of peracetic acid was added dropwise to the reaction bottle at room temperature for 4-5 hours. After the dropwise addition, the temperature was raised to 40-45°C, and the reaction was stirred for 12 hours. After drying, it was concentrated under reduced pressure and spin-dried, and desalted with methanol to obtain 42.4 g of compound III as an oil, w...
Embodiment 2
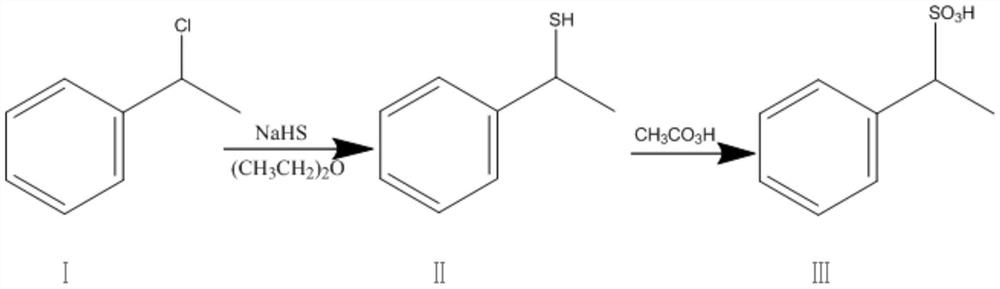
[0036] In a 1000ml four-neck flask equipped with a stirrer, a condenser, and a thermometer, add 100ml of ether, 20.3g of sodium hydrosulfide, start stirring, start to add 34.0g of compound I dropwise, dropwise at a temperature of 25-30°C, dropwise add 1- After 1.5 hours, continue to keep warm for 15 hours after the dropwise addition, controlled by GC, concentrate under reduced pressure after the reaction, recover the organic solvent and use it for the reaction, and the obtained oily substance is compound II. Then, 300.0 g of peracetic acid was added dropwise to the reaction bottle at room temperature for 4-5 hours. After the dropwise addition, the temperature was raised to 40-45°C, and the reaction was stirred for 12 hours. Concentrated under reduced pressure and spin-dried, and desalted with methanol, the obtained oil was 38.5 g of compound III, with a molar yield of 85.6% (calculated according to compound I), and a purity of 98.8%.
[0037]
Embodiment 3
[0039] In a 1000ml four-necked bottle equipped with a stirrer, a condenser, and a thermometer, add 100ml of isopropanol and 20.3g of sodium hydrosulfide, start stirring, and start to add 2.4g of compound I dropwise. The dropping temperature is 30-35°C. 1-1.5 hours, after the dropwise addition, the temperature is raised to 45°C for heat preservation reaction for 10 hours, the heat preservation temperature is 45-50°C, controlled by GC, after the reaction is completed, the reaction is concentrated under reduced pressure, the organic solvent is recovered and the reaction is applied, and the obtained oil is Compound II . Then, 300.0 g of peracetic acid was added dropwise to the reaction bottle at room temperature for 4-5 hours. After the dropwise addition, the temperature was raised to 40-45°C, and the reaction was stirred for 12 hours. Concentrated under reduced pressure and spin-dried, and desalted with methanol, the obtained oil was 45.8 g of compound III, with a molar yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com