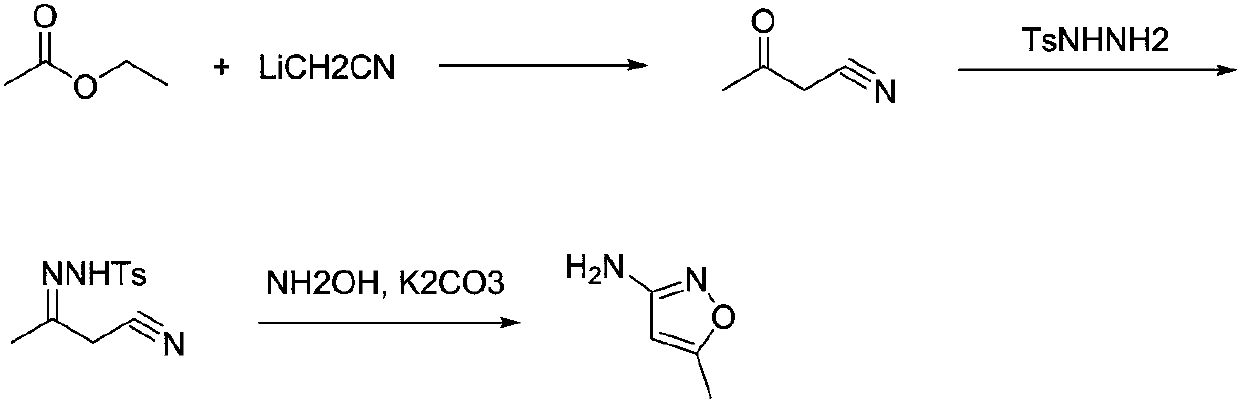
Preparation method for 3-amino-5-methylisoxazole
A technology of methylisoxazole and amino, which is applied in the field of preparation of 3-amino-5-methylisoxazole, which achieves the effects of convenient purchase, transportation and use, reduced labor protection, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The first step, in the 500mL reaction bottle, sodium hydride (12.0g, 0.3mol, 60%) was added in the acetonitrile (10.3g, 0.25mol) solution that was dissolved in 120mL tetrahydrofuran, then added ethyl acetate (26.4g, 0.3mol ), heated to reflux for 4 hours, down to room temperature, quenched with ice water, adjusted to pH 5-6 with 2N HCl, extracted with ethyl acetate, dried the organic layer over anhydrous sodium sulfate, concentrated the solvent to obtain 18.9 g of acetoacetonitrile, GC purity 96%, yield 91%.
[0026] In the second step, in a 1L reaction flask, dissolve the product acetoacetonitrile (18.9g, 0.23mol) obtained in the previous step in 600mL of methanol, add p-toluenesulfonylhydrazide (40.2g, 0.22mol), heat and reflux for 2 hours, and analyze the crystal, filtered to obtain 50.3 g of hydrazone as a white crystalline solid, with an HPLC purity of 99% and a yield of 88%.
[0027] The third step, in the 500mL reaction bottle, add hydroxylamine hydrochloride (1...
Embodiment 2
[0029] In the first step, in a 500mL reaction flask, sodium hydride (14g, 0.35mol, 60%) was added into a solution of acetonitrile (10.3g, 0.25mol) dissolved in 120mL of tetrahydrofuran, and then methyl acetate (25.9g, 0.35mol) was added , heated to reflux for 4 hours, lowered to room temperature, quenched with ice water, adjusted to pH 5-6 with 2N HCl, extracted with ethyl acetate, dried the organic layer over anhydrous sodium sulfate, concentrated the solvent to obtain 19.1 g of acetoacetonitrile, GC purity 96 %, yield 92%.
[0030] In the second step, in a 1L reaction flask, dissolve the product acetoacetonitrile (19.1g, 0.23mol) obtained in the previous step in 600mL ethanol, add p-toluenesulfonyl hydrazide (42.8g, 0.23mol), heat and reflux for 2 hours, and analyze the crystal, filtered to obtain 52.0 g of hydrazone as a white crystalline solid, with an HPLC purity of 99% and a yield of 90%.
[0031] The third step, in the 500mL reaction flask, add hydroxylamine hydrochlor...
Embodiment 3
[0033] In the first step, in a 500mL reaction flask, cool the solution system of diisopropylamine (35.4g, 0.35mol) in tetrahydrofuran (140mL) to below -30°C, and add dropwise a solution of n-butyllithium in n-hexane (140mL, 0.35mol, 2.5M), the dropwise addition was completed, stirred at room temperature for 30 minutes, cooled to -78°C, and acetonitrile (10.3g, 0.25mol) solution of ethyl acetate (30.8g, 0.35mol) was added dropwise, and the dropwise addition was completed. Stir at room temperature for 2 hours, quench with 2N HCl to adjust the pH value to 5-6 after the reaction, extract with ethyl acetate, dry the organic layer with anhydrous sodium sulfate, and concentrate the solvent to obtain 18.1 g of acetoacetonitrile as a colorless oil, with a GC purity of 98% , yield 87%.
[0034] In the second step, in a 1L reaction flask, dissolve 18.1 g of acetoacetonitrile obtained in the previous step in 600 mL of methanol, add p-toluenesulfonyl hydrazide (40.5 g, 0.22 mol), heat to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com