Amino phenoxy substituted phthalocyanine and application thereof to pharmaceutical field
A technology of aminophenoxytetra, phenoxy, which is applied in the field of photothermal agents and photothermal therapy drugs, can solve the problems that have not yet been seen in the application research and limitations of photothermal therapy, and achieve good industrialization prospects, simple operation, Effects that are easy to store
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
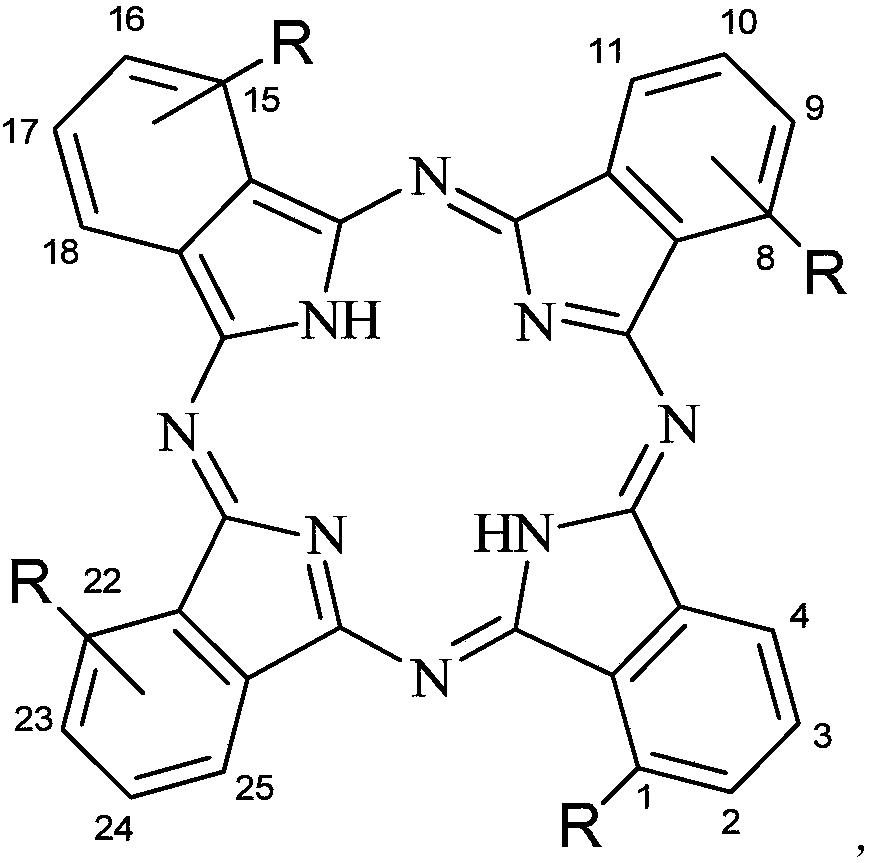
[0065] Synthesis of aminophenoxy tetrasubstituted hollow phthalocyanine with the structure shown in the following formula
[0066] in:
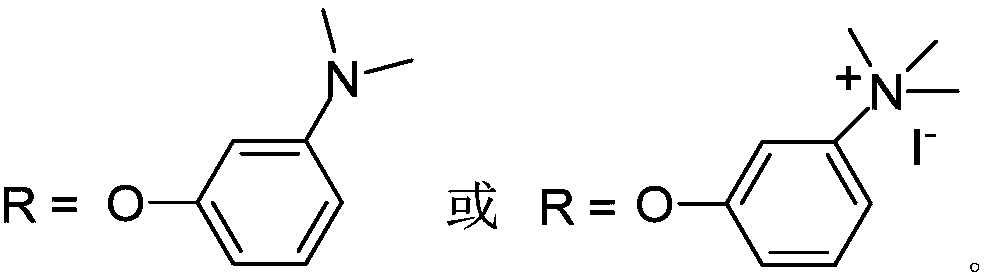
[0067] The compound can be named: Tetra-α-(3-(N,N-dimethylamino)-phenoxy) hollow phthalocyanine, and the preparation method is as follows:
[0068](1) Preparation of (3-(N,N-dimethylamino)-phenoxy)phthalonitrile: Reaction of 3-nitrophthalonitrile and N,N-dimethylm-hydroxyaniline The material, the feed ratio of the two is 1:1~1.5, with dimethyl sulfoxide as solvent, the solvent consumption is that every mmol 3-nitrophthalonitrile needs 3~4mL, in the presence of potassium carbonate and under the protection of nitrogen, Stir the reaction at room temperature to 60°C for 48 to 72 hours. Wherein the dosage of potassium carbonate is 1.5-3 mmol per mmol of 3-nitrophthalonitrile. Monitored by thin-layer chromatography, when 3-nitrophthalonitrile is basically consumed, the reaction is terminated, add water and stand for 4-8 hours, precipitate, d...
Embodiment 2
[0078] Synthesis of aminophenoxy hollow phthalocyanine tetraiodide salt with the structure shown in the following formula
[0079] in:
[0080] The compound can be named: tetra-α-(3-(N,N-dimethylamino)-phenoxy) hollow phthalocyanine tetraiodide salt, and the preparation method is as follows:
[0081] React 0.036 mmol of tetrakis-α-(3-(N,N-dimethylamino)-phenoxy) hollow phthalocyanine described in Example 1 with methyl iodide in an amount of 2.9 to 3.6 mL (preferably 3 mL ), the solvent used is DMF and CHCl 3 The mixed solution (1 / 1) is used in an amount of 2.9-7.2mL (preferably 4mL), and reacted at 25-50°C for 24-48h (preferably 36h). A small amount of DMF was left by rotary evaporation, which was added dropwise to DCM, and a blue precipitate was precipitated, filtered through micropores, washed several times with dichloromethane, and dried in vacuo to obtain a solid with a yield of 89%.
[0082] The characterization data of the product are as follows:
[0083] HRMS(ES...
Embodiment 3
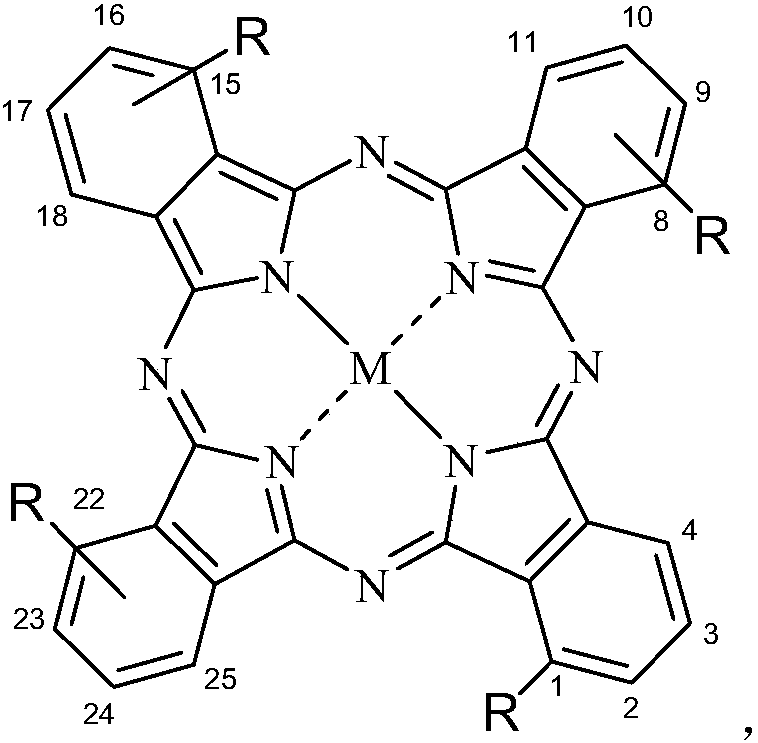
[0086] Synthesis of aminophenoxyphthalocyanine zinc with the structure shown in the following formula
[0087] where M=Zn,
[0088] The compound can be named: tetra-α-(3-(N,N-dimethylamino)-phenoxy)zinc phthalocyanine, and the preparation method is as follows:
[0089] With (3-(N,N-dimethylamino)-phenoxy)phthalonitrile 4mmol as reactant, n-pentanol 32-40mL (preferably 35mL) as solvent, add anhydrous zinc acetate (or other Zinc salt) 1-3mmol (preferably 2mmol), and DBU 0.8-2.4mL (preferably 1.2mL), stirred and reacted at 130-150°C for 12-18 hours, and monitored the reaction end point by thin-layer chromatography. Rotary evaporation, dissolved in dichloromethane, petroleum ether: DCM = 2: 1, passed through a silica gel column, washed away the first reddish-yellow band, then collected the yellow-green band with ethyl acetate, spin-dried, and passed through a tetrahydrofuran gel column, After the collected dark green band was spin-dried, DMF was added to dissolve it, and wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com