Method for determining content of chlortetracycline premix and related substances
A related substance, chlortetracycline technology, applied in the field of analysis and testing, can solve the problems of the influence of content test accuracy, low work efficiency, and low production efficiency, and achieve the goal of improving detection speed and accuracy, reducing inspection cost, and improving detection efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] repeatability test
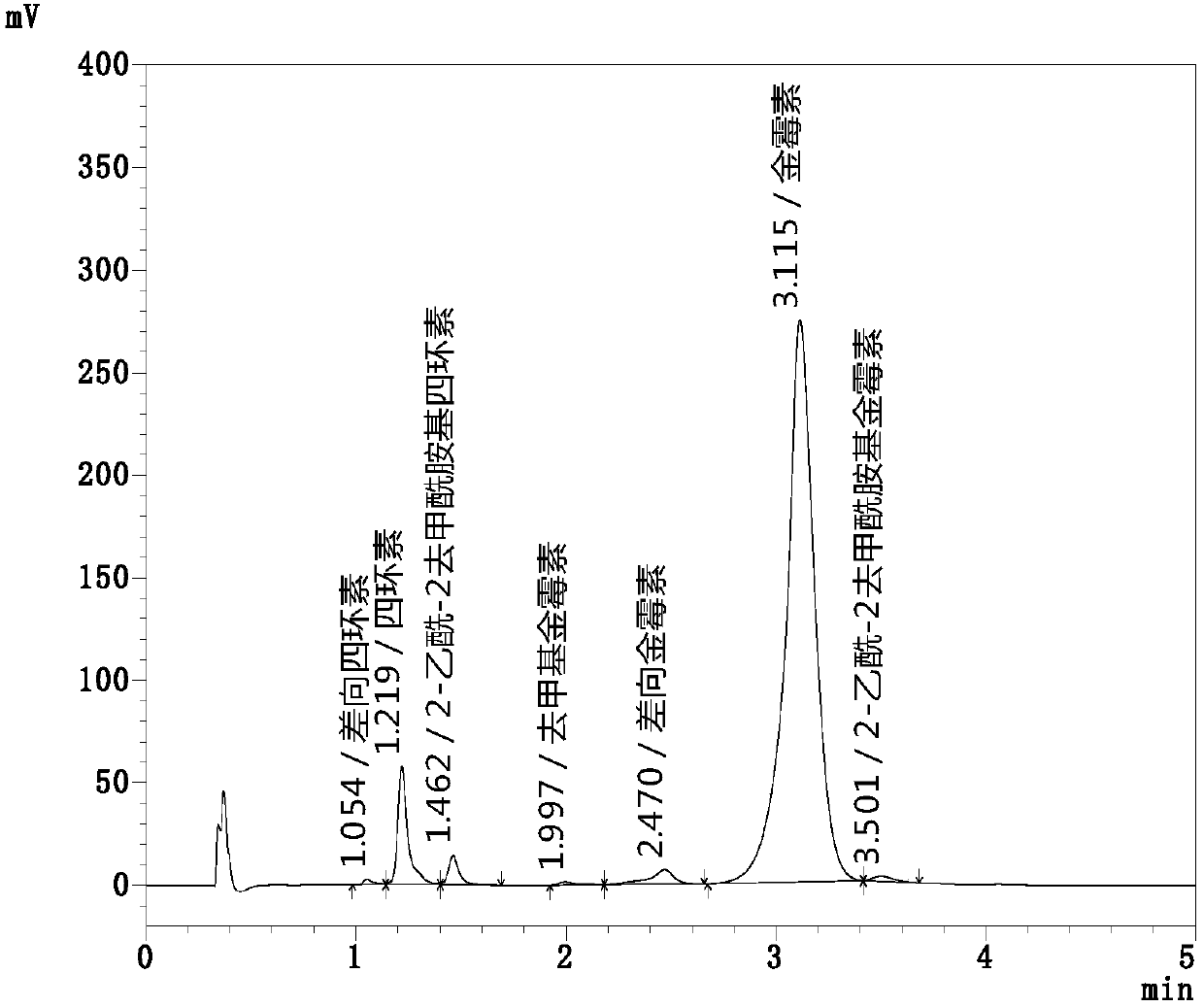
[0060] Get the chlortetracycline test solution of the above-mentioned configuration, inject Shimadzu LC-2040 type ultra-high performance liquid chromatography, carry out high-performance liquid chromatography analysis according to the above-mentioned chromatographic conditions, carry out 6 parallel determinations continuously, and record 6 parallel determinations The peak area of each component, the results are shown in Table 1, wherein the high performance liquid chromatogram of parallel test group 2 is as follows figure 1 shown.
[0061] Table 1 repeatability (system precision) test data statistical table
[0062]
[0063]
[0064] As can be seen from Table 1, the same chlortetracycline need testing solution is measured for 6 consecutive injections, the peak area RSD of chlortetracycline is 0.11%, the peak area RSD of tetracycline is 0.26%, and the RSD of peak area of tetracycline is 1.7%. The peak area RSD of chlortetracycline was 0.4...
Embodiment 2
[0066] Reproducibility test
[0067] Reproducibility, also known as method precision, is determined by testing the same sample on different days with different instruments by different inspectors.
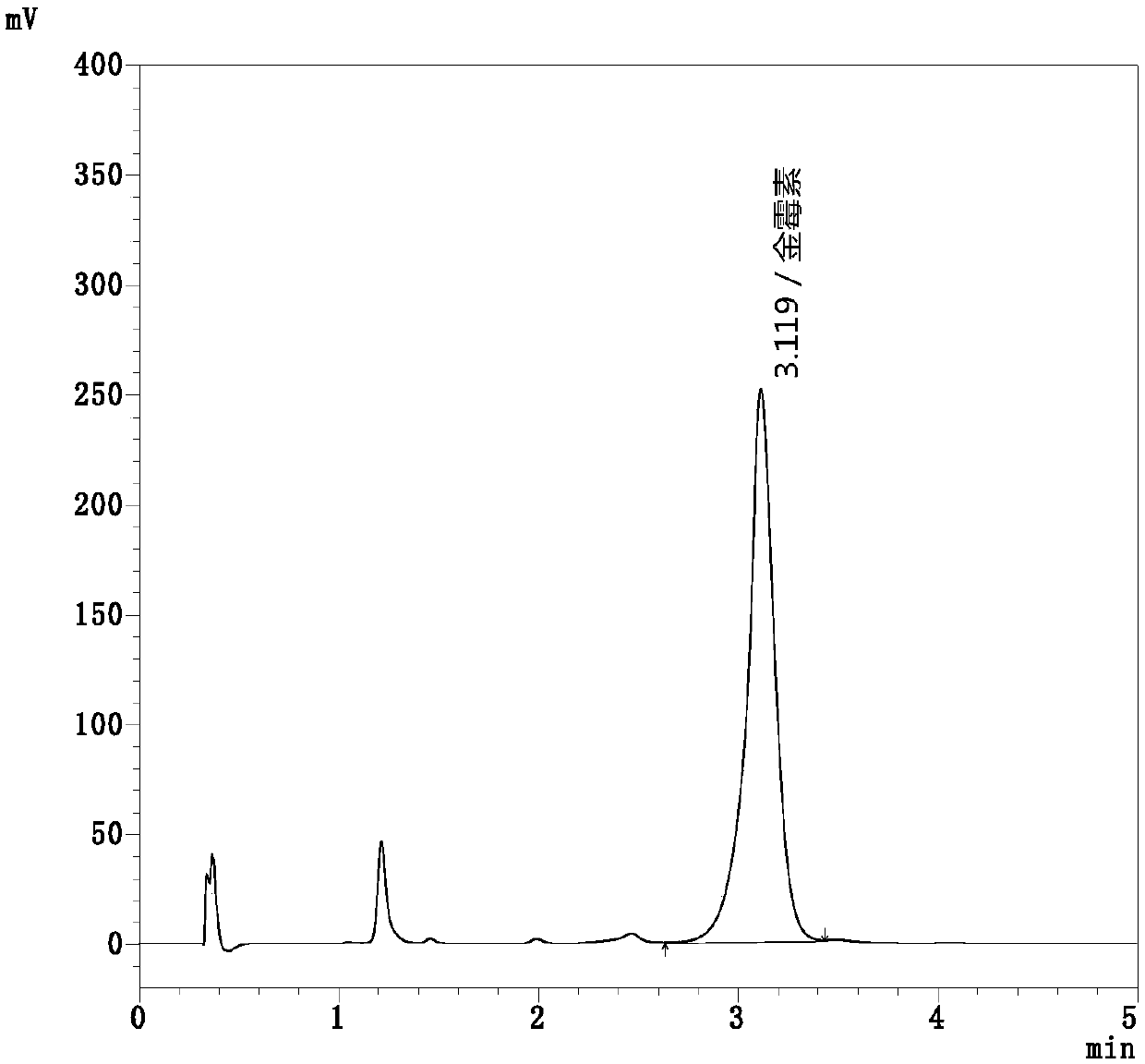
[0068] Get the aureomycin working standard solution of above-mentioned configuration, inject Shimadzu LC-2040 type ultra-high performance liquid chromatograph, carry out high performance liquid chromatography analysis according to above-mentioned chromatographic conditions, obtain aureomycin working standard solution high performance liquid chromatography, Such as figure 2 shown, yes figure 2 The analysis shows that the peak area of aureomycin in the figure is 2424882.
[0069] Take the aureomycin test solution of the above-mentioned configuration, and measure it 6 times by two inspectors on different dates respectively, through the formula: aureomycin in aureomycin premix or aureomycin Carry out the calculation, and record the calculation result, statistically analyze the ...
Embodiment 3
[0075] accuracy test
[0076] Accuracy is the closeness between the measured value and the true value, and the accuracy of the method is usually expressed by the recovery rate. Select a chlortetracycline premix sample of a known specification (the chlortetracycline content is 20.2wt%, tetracycline 1.2wt%, epitrope chlortetracycline 0.51wt%, 2-acetyl-2-decarboxamidotetracycline 0.47 wt%, 2-acetyl-2-descarboxamidomycin 0.22wt%, epitetracycline and demethylchlortetracycline are all less than 0.1wt%), diluted with 0.01mol / L HCl, and prepared three concentration levels Chlortetracycline premix sample solution, 3 parts of each grade parallel determination, carry out 9 times of measurement altogether, then combine the peak area of aureomycin in the working standard solution chromatogram that obtains Chlortetracycline in the embodiment 2, by formula gold Aureomycin and Calculate the content of aureomycin and related substances in each group, and then calculate the recovery rate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com