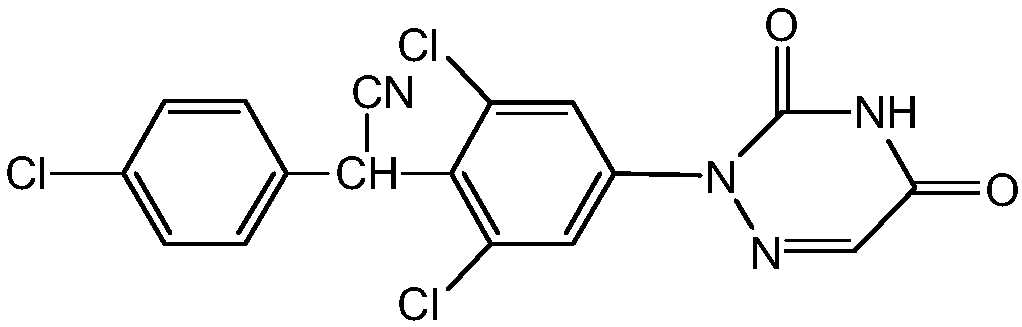
Preparation method of anticoccidial drug Diclazuril
A technology of diclazuril and anticoccidiostats, applied in the field of drug synthesis, to achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] Preparation of 3,4,5-trichloronitrobenzene
[0022] Put 62.5 mL of concentrated sulfuric acid into a 250 mL four-necked flask, and add 11.5 g of sodium nitrite under stirring. The temperature was raised to 70°C and stirred for 10 minutes, then the temperature was reduced to 55°C, 31.2g of 2,6-dichloro-4-nitroaniline was slowly added, and the temperature was lowered to 5°C after 20 minutes of holding. The diazonium solution prepared by adding 40 mL of acetic acid and keeping the temperature for 30 minutes.
[0023] Add 125 mL of 36.5% hydrochloric acid and 15 g of cuprous chloride to another 250 mL four-necked flask. After stirring for 15 minutes, the above diazonium liquid is slowly added dropwise. The temperature during the dropping process is controlled below 25°C. After the dripping, the temperature was raised to 70°C and kept for 1 hour. After the incubation, the temperature is lowered to room temperature and filtered, and washed with 1% lye. The brown crystals obtai...
Embodiment 1
[0025] Preparation of 2,6-Dichloro-α-(4-chlorophenyl)-4-nitrobenzeneacetonitrile
[0026] Into a 500 mL four-necked flask, 170 mL of 2-butanone, 55 g of 3,4,5-trichloronitrobenzene, and 40.5 g of p-chlorobenzene acetonitrile were placed, and the temperature was raised to 50°C. 50% sodium hydroxide solution was added dropwise, and the reaction was kept for 2h after the dropping. After the incubation, the temperature was lowered to 25°C and hydrochloric acid was added dropwise to adjust pH=2. After adding 70 mL of water, the temperature was raised to 50° C. to separate layers. The water layer was removed, and the organic layer was distilled under reduced pressure to collect 2-butanone for recycling. Methanol was added and the temperature was reduced to 20° C., and 74.7 g of light yellow condensate powder was obtained by filtration. The yield was 90%, and the content was 99.59% (HPLC).
[0027] Preparation of 2,6-Dichloro-α-(4-chlorophenyl)-4-aminobenzeneacetonitrile
[0028] Into a...
Embodiment 2
[0032] Preparation of 2,6-Dichloro-α-(4-chlorophenyl)-4-nitrobenzeneacetonitrile
[0033] Into a 500 mL four-necked flask, 200 mL of 2-butanone, 55 g of 3,4,5-trichloronitrobenzene, and 42.4 g of p-chlorobenzene acetonitrile were placed, and the temperature was raised to 50°C. 30% sodium hydroxide solution was added dropwise, and the reaction was kept for 3 hours after the dropping. After the incubation, the temperature was lowered to 30°C and hydrochloric acid was added dropwise to adjust the pH=1. After adding 55 mL of water, the temperature was raised to 55°C to separate layers. The water layer was removed, and the organic layer was distilled under reduced pressure to collect 2-butanone for recycling. The temperature was lowered to 20°C by adding methanol, and 73.04 g of light yellow condensate powder was obtained by filtration, the yield was 88%, and the content was 99.3% (HPLC).
[0034] Preparation of 2,6-Dichloro-α-(4-chlorophenyl)-4-aminobenzeneacetonitrile
[0035] Into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com