Cinnamaldehyde-based polymer with acid and photo-degradation properties and preparation method thereof
A technology of cinnamaldehyde and polymer, applied in the field of polymer chemistry, can solve problems such as restricting the application of polymer materials, and achieve the effects of low difficulty in operation method, mild reaction conditions and wide application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
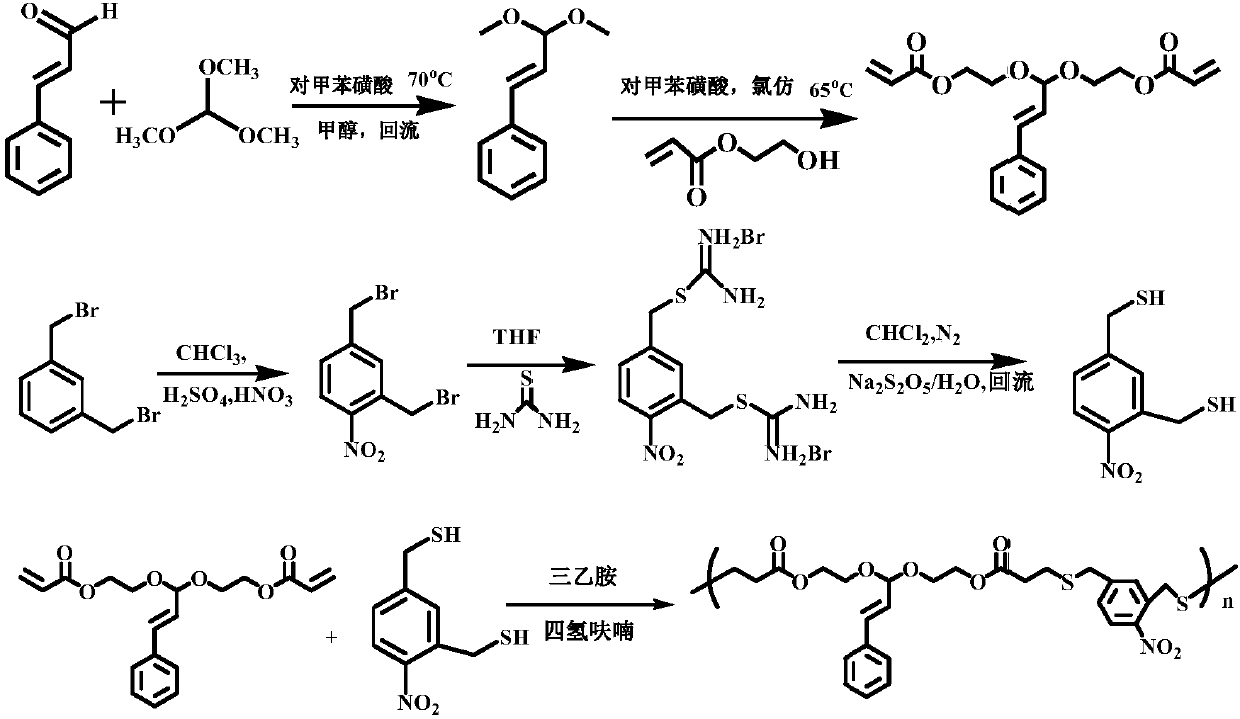
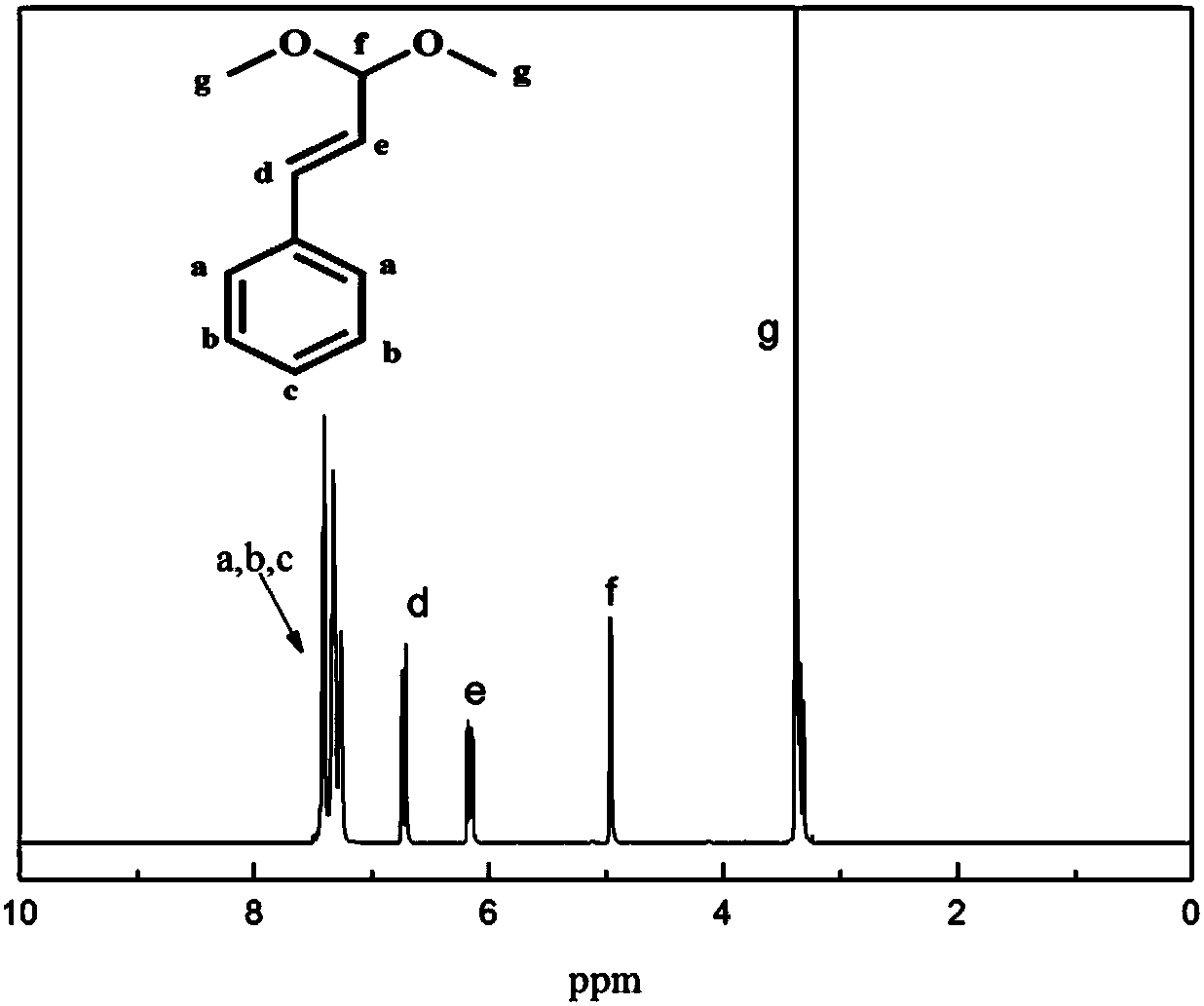
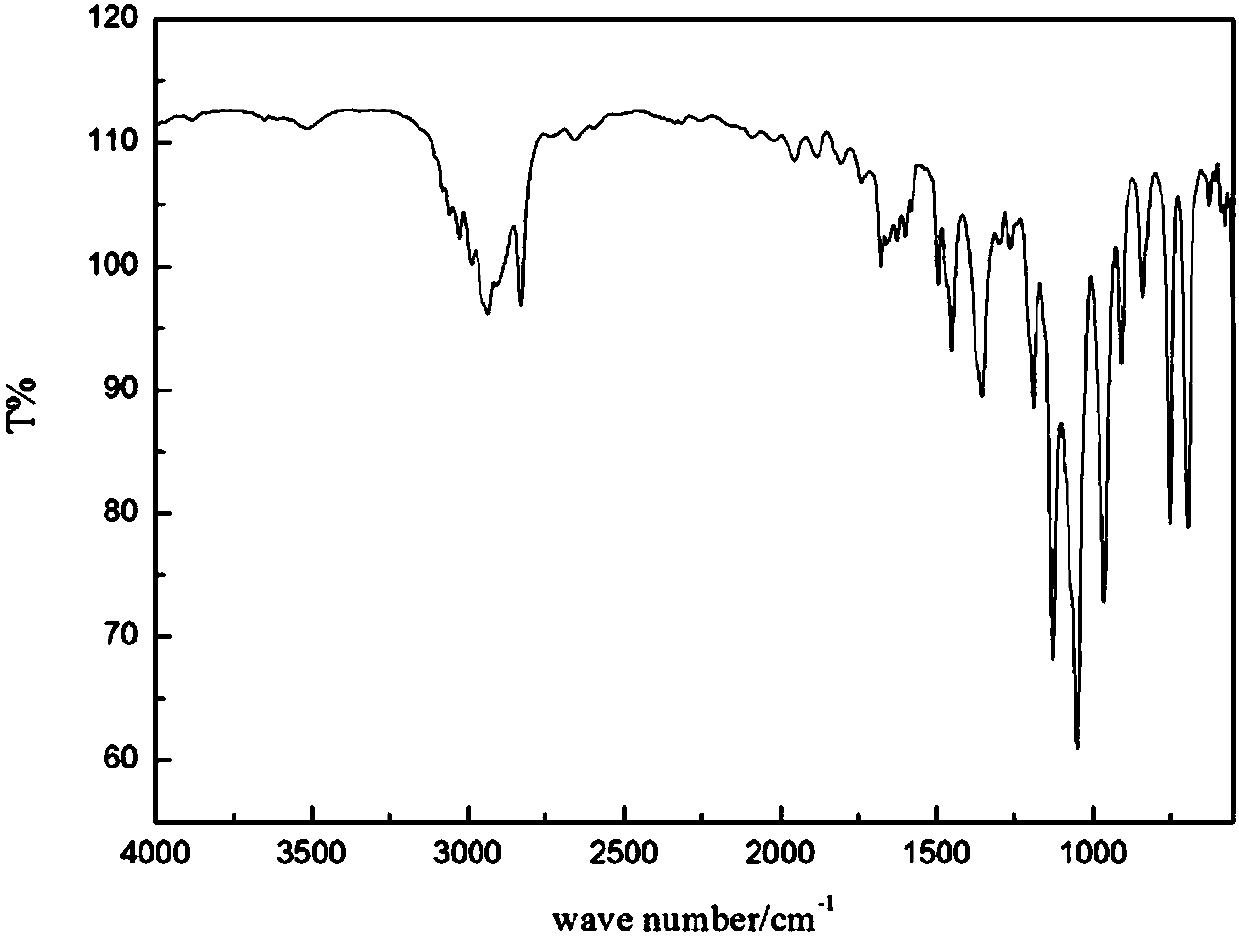
[0054] Such as Figure 1 to Figure 5 As shown, in Embodiment 1 of the present invention, the following steps are included:
[0055] (1) At room temperature, weigh 6.6g (0.05mol) of cinnamaldehyde and 23.8g (0.22mol) of trimethyl orthoformate in a dry and clean round bottom flask, dissolve them in 60mL of methanol, add 30mg of p-toluenesulfonic acid , the reaction system was refluxed at 72°C and 800r / min for 6 hours;
[0056] (2) Add saturated aqueous sodium bicarbonate solution to the reaction system in batches to pH = 7-8, remove p-toluenesulfonic acid, concentrate under reduced pressure, extract 3 times with 3*40mL dichloromethane, collect the organic layer, add 8g of anhydrous Sodium sulfate dehydration for 6 hours;
[0057] (3) Remove anhydrous sodium sulfate by filtration, remove the solvent under reduced pressure, and separate by column chromatography. The volume ratio of the column chromatography components is petroleum ether: ethyl acetate=10:1, and the light yellow li...
Embodiment 2
[0076] (1) At room temperature, weigh 3.3g (0.025mol) of cinnamaldehyde and 9.9g (0.0.092mol) of trimethyl orthoformate in a dry and clean round bottom flask, dissolve them in 30mL of methanol, add 12mg of p-toluenesulfonate acid, the reaction system was refluxed at 68°C and 800r / min for 4 hours;
[0077] (2) Add saturated aqueous sodium bicarbonate solution to the reaction system in batches to pH = 7-8, remove p-toluenesulfonic acid, concentrate under reduced pressure, extract 3 times with 3*20mL dichloromethane, collect the organic layer, add 4g of anhydrous Sodium sulfate dehydration for 4 hours;
[0078] (3) Remove anhydrous sodium sulfate by filtration, remove the solvent under reduced pressure, separate by column chromatography, the volume ratio of the column chromatography components, petroleum ether: ethyl acetate=10:1, to obtain a light yellow liquid cinnamon acetal 3.738 g, yield 84%;
[0079] (4) Dissolve 3.73 g (0.021 mol) of cinnamon acetal obtained in step (3) ...
Embodiment 3
[0084] (1) At room temperature, weigh 5g of cinnamaldehyde and 25g of trimethyl orthoformate in a dry and clean round bottom flask, dissolve them in 90mL of methanol, add 45mg of p-toluenesulfonic acid, and the reaction system is at 80°C, 800r / min Under reflux reaction for 8 hours;
[0085] (2) Add saturated aqueous sodium bicarbonate solution to the reaction system in batches to pH = 7‐8, remove p-toluenesulfonic acid, concentrate under reduced pressure, extract 3 times with 3*30mL dichloromethane, collect the organic layer, add 10g of anhydrous Sodium sulfate dehydration for 6 hours;
[0086] (3) Remove anhydrous sodium sulfate by filtration, remove the solvent under reduced pressure, and separate by column chromatography. The volume ratio of the column chromatography components is petroleum ether: ethyl acetate=10:1, and the light yellow liquid cinnamon acetal 5.40 is obtained. g, yield 80%;
[0087] (4) Dissolve 5.4g of cinnamon acetal and 14g of hydroxyethyl acrylate ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com