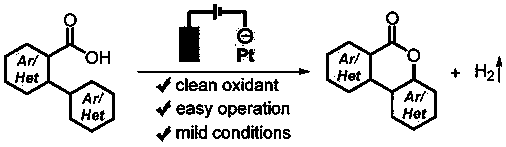
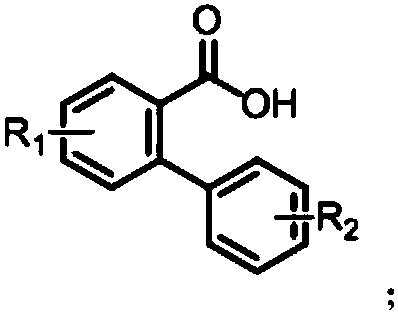
Synthesis method of polyaryls six-membered ring lactone compound
A technology of ester compounds and aryl six-membered rings, which is applied in the field of organic compound synthesis to achieve the effects of mild conditions, wide substrate applicability, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Add 2-phenylbenzoic acid (0.5mmol) and lithium perchlorate (1.0mmol) to a 25mL reaction tube equipped with a magnetic stirrer, then add 10.0mL acetonitrile; fix the reaction tube on a magnetic stirrer, add The electrodes (platinum sheet cathode, graphite anode) were electrolyzed with a 6mA constant current, and the reaction solution was uniformly stirred at the same time; after the mixture was reacted at room temperature (25°C) for 5h, the reaction was completed; the solvent was removed with a rotary evaporator, and the crude product was subjected to column chromatography ( Petroleum ether:ethyl acetate=20:1, v / v) The target product (2a) was obtained by separation and purification with a yield of 85%. The NMR data of this compound are: 1 H NMR (400MHz, CDCl 3 )δ8.38(dd, J=7.9,0.9Hz,1H),8.09(d,J=8.1Hz,1H),8.03(dd,J=7.9,1.3Hz,1H),7.89–7.73(m,1H ),7.67–7.53(m,1H),7.47(ddd,J=8.4,7.3,1.5Hz,1H),7.33(ddd,J=11.3,6.5,2.3Hz,2H). 13 C NMR (101MHz, CDCl 3 )δ161.16, ...
Embodiment 2
[0030]
[0031] 4'-methyl-[1,1'-biphenyl]-2-carboxylic acid (1b) was used instead of 2-phenylbenzoic acid (1a), and the others were the same as in Example 1. Column chromatography (petroleum ether: ethyl acetate = 20:1, v / v) gave the target product (2b) in a yield of 88%. The NMR data of this compound are: 1 H NMR (400MHz, CDCl 3 )δ8.37(dd, J=8.0,1.0Hz,1H),8.07(d,J=8.1Hz,1H),7.92(d,J=7.9Hz,1H),7.86–7.73(m,1H), 7.64–7.43(m,1H),7.14(d,J=8.7Hz,2H). 13 C NMR (101MHz, CDCl 3 )δ161.44, 151.24, 141.28, 134.97, 134.77, 130.51, 128.35, 125.66, 122.49, 121.42, 120.84, 117.86, 115.40, 21.44.
Embodiment 3
[0033]
[0034] 4'-fluoro-[1,1'-biphenyl]-2-carboxylic acid (1c) was used instead of 2-phenylbenzoic acid (1a), and the others were the same as in Example 1. Column chromatography (petroleum ether:ethyl acetate=20:1, v / v) gave the target product (2c) in a yield of 93%. The NMR data of this compound are: 1 H NMR (400MHz, CDCl 3 )δ8.34(dd, J=8.0,1.0Hz,1H),8.00(dd,J=8.3,5.6Hz,2H),7.86–7.74(m,1H),7.61–7.48(m,1H),7.12 –6.97(m,2H). 13 C NMR (101MHz, CDCl 3 )δ163.41(d, J=251.3Hz), 160.75, 152.08(d, J=12.3Hz), 135.07, 134.16, 130.61, 128.74, 124.34(d, J=9.9Hz), 121.48, 120.35, 114.56(d , J=3.2Hz), 112.42(d, J=22.4Hz), 105.03(d, J=25.3Hz). 19 F NMR (376MHz, CDCl 3 )δ-108.36(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com