Luminescent transparent glass containing europium ion self-reducing ability and preparation method thereof
A technology of luminescent glass and europium ions, applied in glass manufacturing equipment, glass molding, manufacturing tools, etc., can solve the problems of reducing the light decay of phosphor powder, easy aging of epoxy resin, complicated packaging process, etc., and achieve uniform texture and reduction. The effect of high degree and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0027] 1) Weigh the raw material BaCO according to the molar ratio of 1:1:1:0.01:y:0.05:0.28 (where y=0, 0.28, 0.55, 0.83) 3 , ZnO, SiO 2 , Eu 2 o 3 , H 3 BO 3 , NH 4 h 2 PO 4 , Na 2 SO 4 , the weighed raw materials were ground in an agate mortar for 30 minutes at room temperature, and mixed evenly to obtain a glass precursor for later use.
[0028] 2) Put the precursor in step 1) into a corundum crucible, put the precursor together with the crucible in a silicon-molybdenum furnace, keep it at 1400°C for 2 hours, and wait for it to melt and homogenize into glass liquid. After taking out the molten glass, pour it into a graphite mold to form it, then move it into a preheated resistance furnace, anneal at 600°C for 30 min, and naturally cool to room temperature with the furnace temperature to obtain the initial sample.
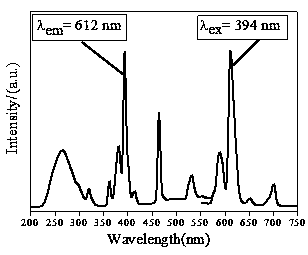
[0029] 3) Part of the initial sample obtained in step 2) was cut and polished, and part of it was ground into powder to test its luminescent propertie...
Embodiment example 2
[0032] 1) Weigh the raw material BaCO according to the molar ratio of 1:1:1:0.01:0.83:z:0.28 (where z=0, 0.01, 0.02, 0.03, 0.04, 0.05, 0.06) 3 , ZnO, SiO 2 , Eu 2 o 3 , H 3 BO 3 , NH 4 h 2 PO 4 , Na 2 SO 4 , the weighed raw materials were ground in an agate mortar for 30 minutes at room temperature, and mixed evenly to obtain a glass precursor for later use.
[0033] 2) Put the precursor in step 1) into a corundum crucible, put the precursor together with the crucible in a silicon-molybdenum furnace, keep it at 1400°C for 2 hours, and wait for it to melt and homogenize into glass liquid. After taking out the molten glass, pour it into a graphite mold to form it, then move it into a preheated resistance furnace, anneal at 600°C for 30 min, and naturally cool to room temperature with the furnace temperature to obtain the initial sample.
[0034] 3) Part of the initial sample obtained in step 2) was cut and polished, and part of it was ground into powder to test its lum...
Embodiment example 3
[0041] 1) Weigh the raw material BaCO according to the molar ratio of 1:1:1:x:0.83:0.05:0.28 (where x=0.01, 0.02, 0.03, 0.04, 0.05) 3 , ZnO, SiO 2 , Eu 2 o 3 , H 3 BO 3 , NH 4 h 2 PO 4 , Na 2 SO 4 , the weighed raw materials were ground in an agate mortar for 30 minutes at room temperature, and mixed evenly to obtain a glass precursor for later use.
[0042] 2) Put the precursor in step 1) into a corundum crucible, put the precursor together with the crucible in a silicon-molybdenum furnace, keep it at 1400°C for 2 hours, and wait for it to melt and homogenize into glass liquid. After taking out the molten glass, pour it into a graphite mold to form it, then move it into a preheated resistance furnace, anneal at 600°C for 30 min, and naturally cool to room temperature with the furnace temperature to obtain the initial sample.
[0043] 3) Part of the initial sample obtained in step 2) was cut and polished, and part of it was ground into powder to test its luminescent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
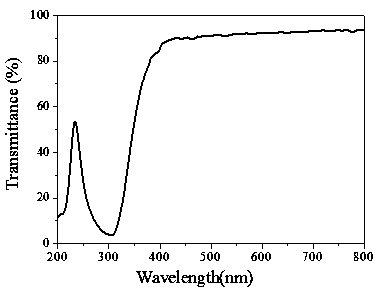
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com