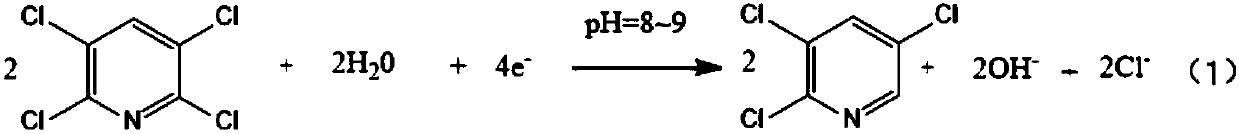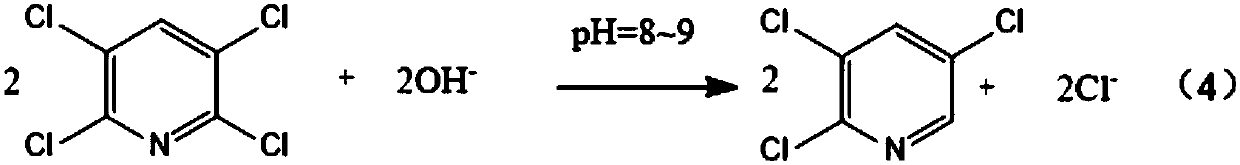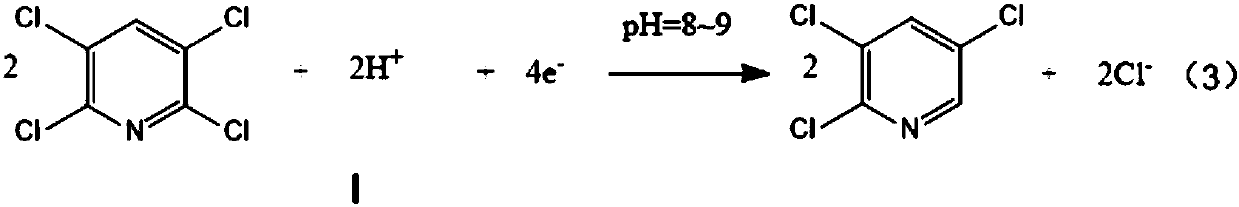Preparation method of 2,3,5-trichloropyridine
A technology of trichloropyridine and tetrachloropyridine, which is applied in the field of preparation of 2,3,5-trichloropyridine, achieves the effects of short reaction time, non-corrosion, and reduced treatment cost of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The diaphragm plate frame tank is an electrolytic reactor, the perfluorosulfonic acid membrane is a diaphragm, the lead sheet is a cathode, and the graphite plate is an anode. 1000mL 0.1mol / L 2,3,5,6-tetrachloropyridine (TeCP) (21.8g, GC content 99.1%)+0.3mol / L NH 4 The methanol solution of Cl (16.2g) + 1% vol ammonia water (ammonia content 25% ~ 28%) + 9% vol water is the catholyte; 1000mL0.1mol / L sodium hydroxide aqueous solution is the anolyte. During the electrolysis process, the temperature is controlled at 45-50°C, and the current density is controlled at 3A / dm 2 , the initial pH of catholyte=8. Stop electrolysis after electrifying for 4.2h (that is, a theoretical electric quantity Q*). GC analysis of the catholyte showed:
[0054] 3,5-Dichloropyridine: 7.671%
[0055] 2,5-Dichloropyridine: 4.238%
[0056] 2,3,5-Trichloropyridine: 60.46%
[0057] 2,3,6-Trichloropyridine: 5.152%
[0058] 2,3,5,6-Tetrachloropyridine: 21.38%
[0059] After distilling the cath...
Embodiment 2
[0062] Same operation with embodiment 1, but change anolyte sodium hydroxide concentration into 0.15mol / L, the GC analysis of catholyte shows:
[0063] 3,5-Dichloropyridine: 5.405%
[0064] 2,5-Dichloropyridine: 3.139%
[0065] 2,3,5-Trichloropyridine: 56.91%
[0066] 2,3,6-Trichloropyridine: 5.143%
[0067] 2,3,5,6-Tetrachloropyridine: 28.46%
[0068] The products obtained by the comprehensive treatment of the reaction solution are respectively:
[0069] 0.99g of 3,5-dichloropyridine, 0.66g of 2,5-dichloropyridine, 12.11g of 2,3,5-trichloropyridine, 1.03g of 2,3,6-trichloropyridine and unreacted 2,3 , 5,6-tetrachloropyridine 5.96g, in summary, the yield of 2,3,5-trichloropyridine is 55.55%.
Embodiment 3
[0071] Same operation as embodiment 1, but ammoniacal liquor is changed into triethylamine, and the GC analysis of catholyte shows:
[0072] 3-chloropyridine: 5.498%
[0073] 2,5-Dichloropyridine: 3.452%
[0074] 2,3,5-Trichloropyridine: 70.24%
[0075] 2,3,6-Trichloropyridine: 5.795%
[0076] 2,3,5,6-Tetrachloropyridine: 14.43%
[0077] The products obtained by the comprehensive treatment of the reaction solution are respectively:
[0078] 0.83g of 3-chloropyridine, 0.58g of 2,5-dichloropyridine, 14.43g of 2,3,5-trichloropyridine, 1.02g of 2,3,6-trichloropyridine and unreacted 2,3,5, 3.06 g of 6-tetrachloropyridine. In summary, the yield of 2,3,5-trichloropyridine is 66.19%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com