Alkylation method for isobutane and C3-C5 olefins
A technology of alkene alkyl and isobutane is applied in the field of alkylation reaction between isobutane and C3-C5 alkene, and can solve the problems of low octane number of reaction products, short alkylation reaction time, incomplete reaction and the like, To achieve the effect of being beneficial to the octane number of the product, the reaction is sufficient, and the octane number is improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
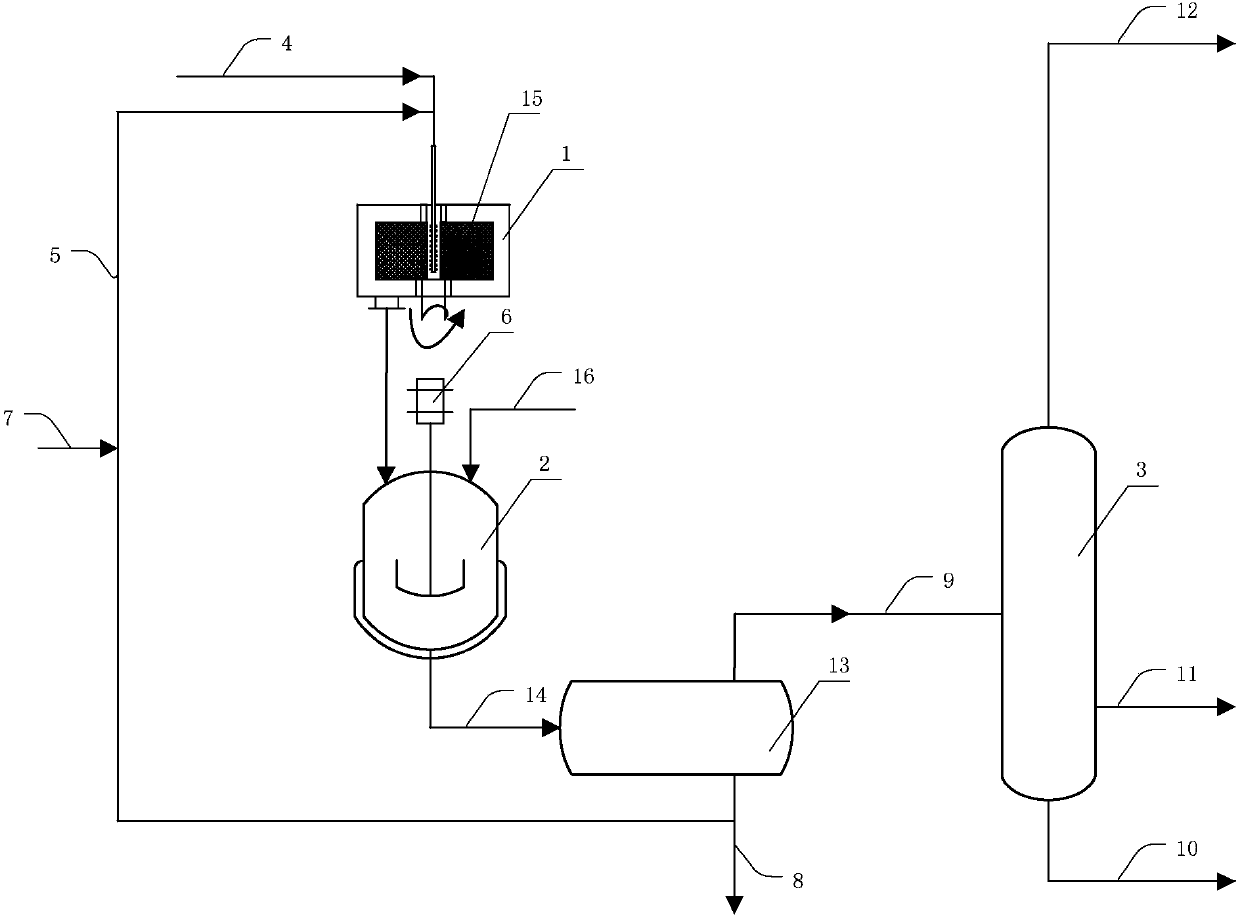
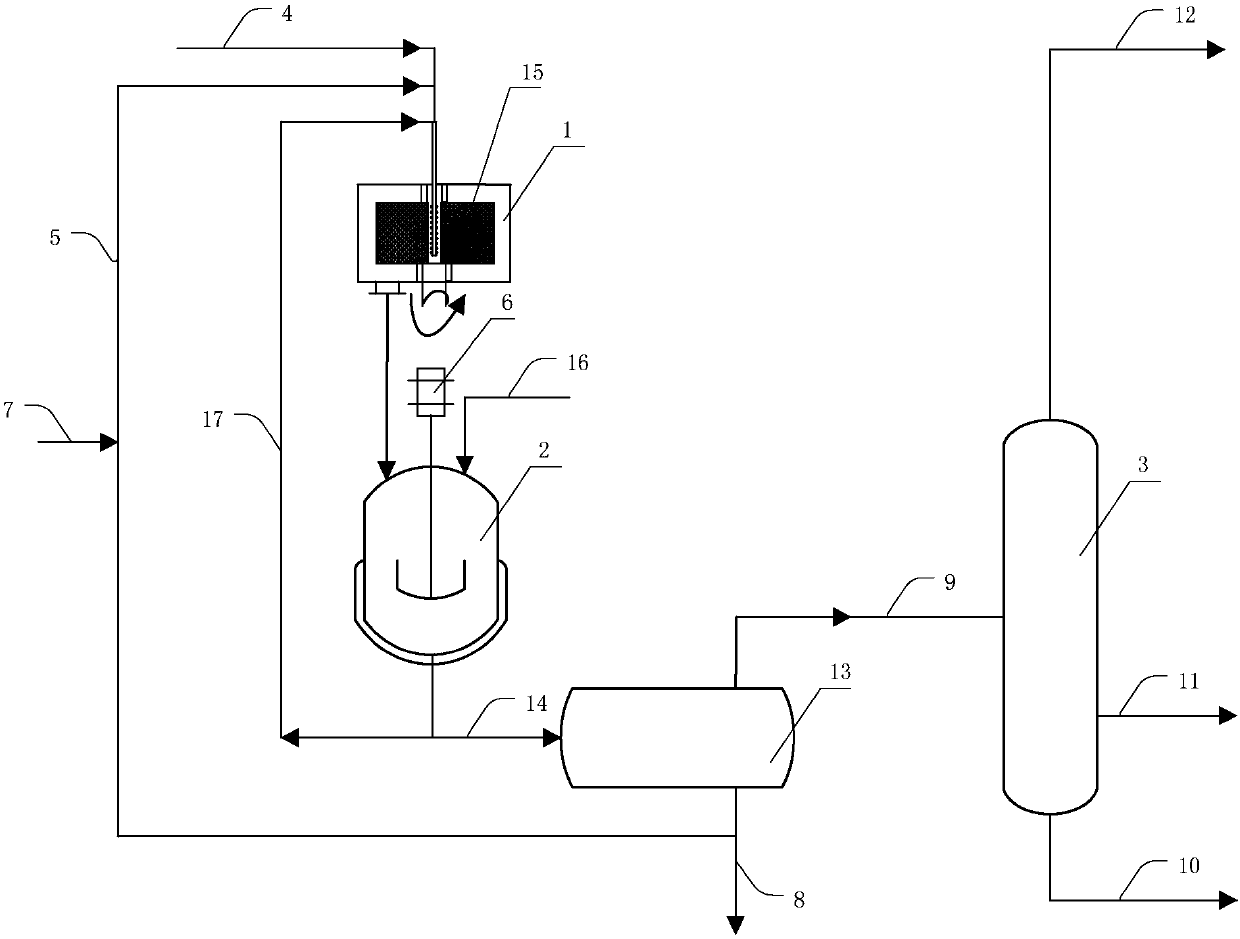
[0050] device see figure 1shown. The raw material hydrocarbon and catalyst sulfuric acid enter the rotary packed bed 1 through the delivery pipe 4 respectively, and are mixed in the rotary packed bed filler 15 to form the acid hydrocarbon emulsion of the raw material hydrocarbon and sulfuric acid, and start to react, wherein the rotary packed bed is the Liquid-liquid dispersion device. Then, it is thrown out under the action of the centrifugal force of the rotating packing, and the reaction material formed of unreacted raw material hydrocarbon, product and sulfuric acid is formed at the outlet of the rotating packed bed. The reaction materials enter the stirred reactor 2 to continue the reaction. The stirred reactor is driven by the motor 6, and the pressure of the stirred reactor is adjusted by filling nitrogen, methane, ethane and other gases through the gas-filling tube 16. The reaction material of the stirring reactor enters the acid hydrocarbon separator 13 through the ...
Embodiment 2
[0063] The difference from Example 1 is that the pressure of the stirred reactor is 0.31 MPa by filling nitrogen gas, the stirring blade is a pitched paddle, and the ratio of the diameter of the paddle to the diameter of the stirred reactor is 1 / 3, and the others are the same as in Example 1. The resulting alkylate had an octane number of 97.2 (research octane number).
Embodiment 3
[0065] The device and operation flow are the same as in Example 1. The molar ratio of isobutane to olefin in the raw material hydrocarbon is 10:1, wherein the olefin is 1-butene, the reaction temperature of the rotating packed bed is 3° C., and the dispersed phase of the acid hydrocarbon emulsion is averaged The diameter is 4.9 μm, the reaction temperature of the stirred reactor is 10°C, the stirring impeller is a bifold paddle, the ratio of the diameter of the paddle to the diameter of the stirred reactor is 1 / 4, and the pressure of the stirred reactor is 0.3 MPa (reaction material The bubble point pressure is 0.2MPa), the average residence time of the reaction material in the stirred reactor is 60min, and the volume ratio of the liquid hydrocarbon in the reaction material to the catalyst sulfuric acid is 1:1.2. The resulting alkylate had an octane number of 97.1 (research octane number).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com