Nonmetal catalyst used for selective oxidation of methane, optimization method and application thereof
A non-metallic catalyst and catalyst technology, applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, non-metallic elements, etc., can solve the problems of easy over-oxidation, scarce reserves, high price, etc., and achieve the effect of improving catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
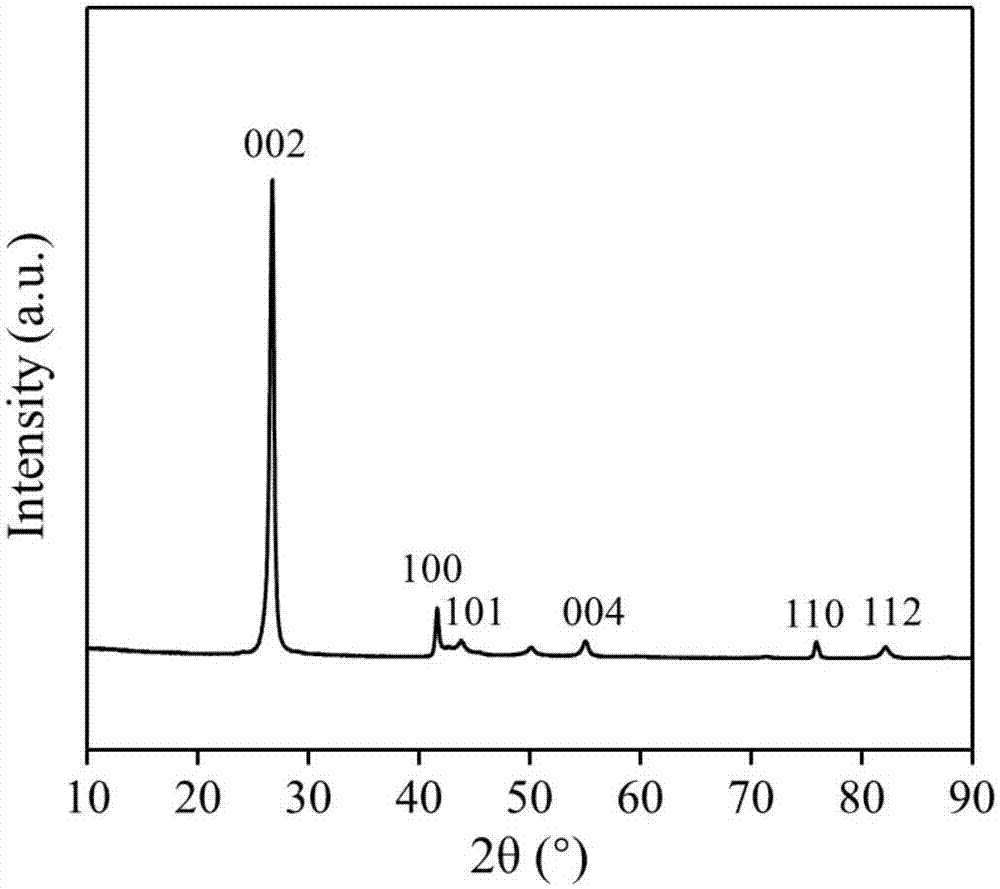
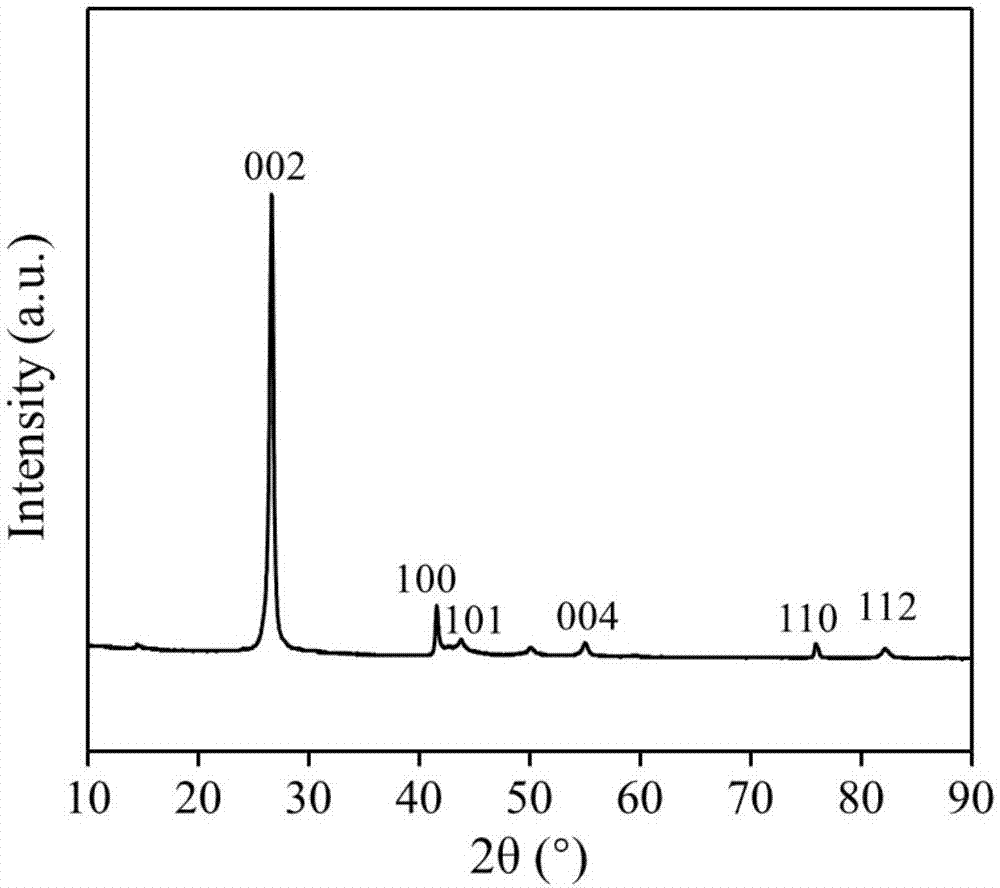
[0032] Direct application of unactivated commercial hexagonal boron nitride for partial oxidation of methane.
[0033] The catalyst was evaluated in an isothermal fixed-bed reactor, and the evaluation process was briefly described as follows:
[0034] The mixed gas of methane, oxygen and diluent in different volume ratios is regulated by a mass flow meter, and the oxidation reaction is carried out in a quartz reaction tube with an inner diameter of 8 mm and a length of 400 mm. The reacted gas enters the gas chromatograph to analyze the composition after passing through the cold trap.
[0035] Methane conversion and product selectivity were calculated according to the following formula:
[0036]
[0037]
[0038]
[0039] Weigh 0.1 g of the catalyst for methane oxidation conversion reaction test. The feed gas volume ratio is CH 4 :O 2 :N 2 =2:1:4, temperature 640~740°C, normal pressure reaction, test results are shown in Table 1.
Embodiment 2
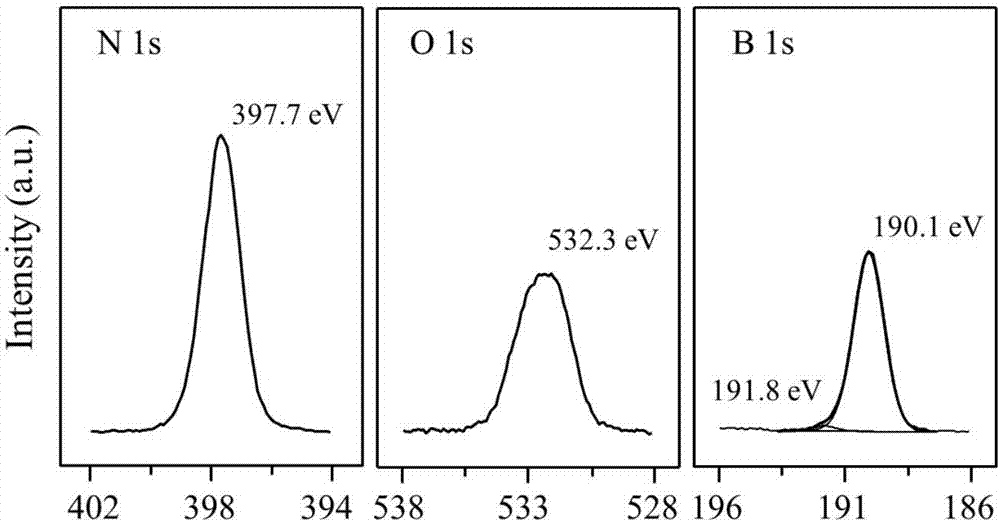
[0041]The boron nitride powder was ball-milled in air atmosphere for 4 hours, and the ball-milled sample was treated in air or oxygen atmosphere at 700°C for 2 hours; the obtained material was activated at 700°C in a humid air atmosphere for 6 hours. Then wash with ammonia water and deionized water to remove impurities existing in the material and impurities introduced during the treatment process to obtain a functionalized catalyst.
[0042] The catalyst testing procedure is the same as in Example 1.
Embodiment 3~5
[0043] Embodiment 3~5, press the operating procedure of embodiment 1, change CH 4 / O 2 The corresponding parameters are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com