Catalyst for oxidative dehydrogenation of light alkane to prepare olefin, optimization method and application thereof
A technology for oxidative dehydrogenation of low-carbon alkanes, applied in physical/chemical process catalysts, chemical instruments and methods, hydrocarbons, etc., to achieve the effects of less CO2 emissions, high conversion rate of low-carbon alkanes, and improved catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
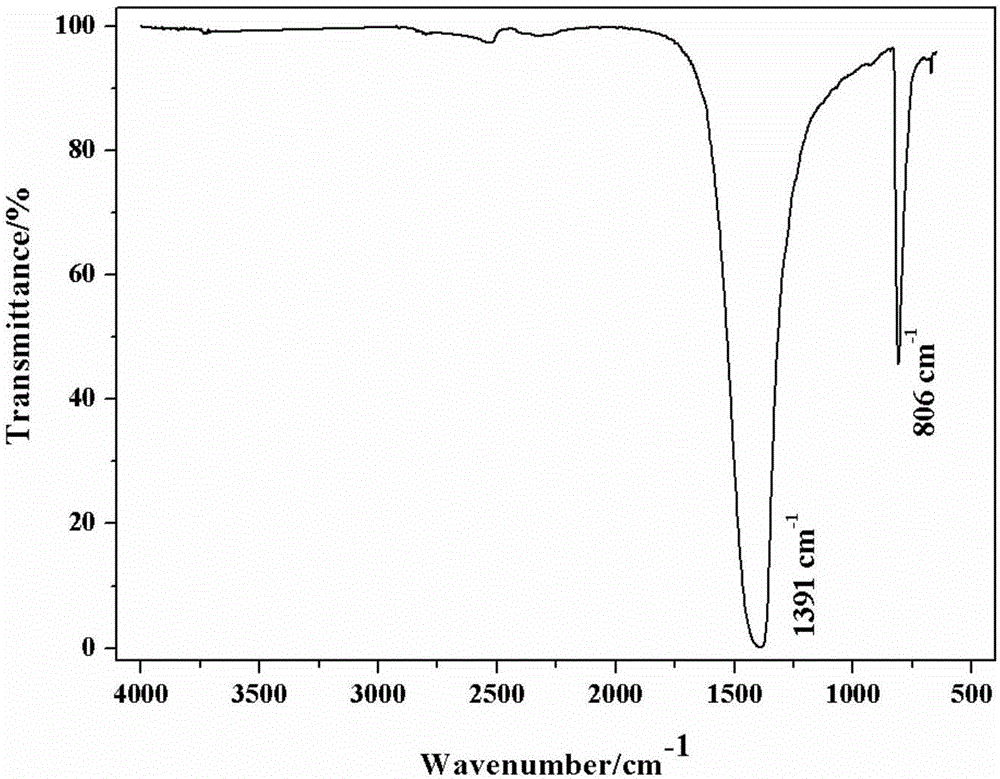
[0034] Add 6.916g of bulk boron nitride powder and 4.58g of urea, the molar ratio of boron nitride and urea is 1:5, put them into a ball mill jar, and mill for 6 hours alternately. After ball milling, add 184ml deionized water to dissolve and disperse, and ultrasonicate in water bath for 2h. The liquid mixture after ultrasound is transferred to a dialysis zone for dialysis to remove urea, and then centrifuged at 3000 rpm for 30 minutes, and the obtained liquid mixture is dried overnight in an oven to obtain boron nitride nanosheets.
Embodiment 2
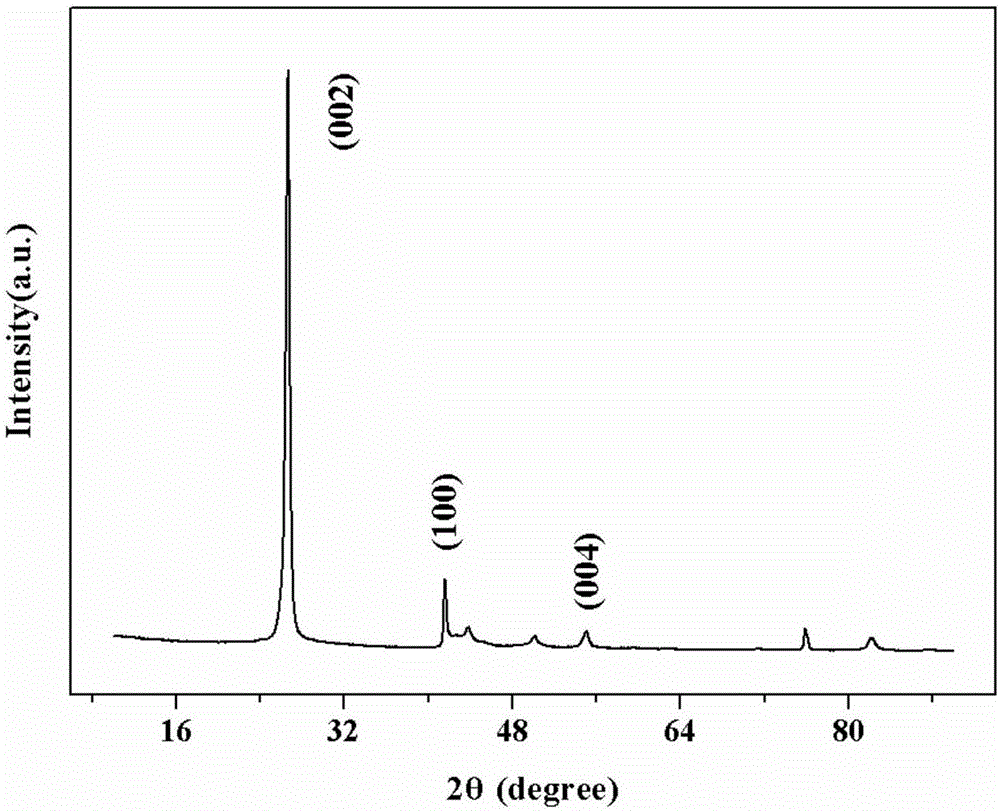
[0036] Add 0.63g of melamine, 0.45g of urea and 1.86g of boric acid into a ball mill jar for ball milling for 2 hours. The molar ratio of nitrogen atoms to boron atoms is 1.5:1, and the molar ratio of melamine to urea nitrogen atoms is 1:0.5. Add 10ml of ethanol to dissolve the raw material, and then evaporate until the residual solvent is 0.5mL. Transfer the wet material to a tube furnace, react at 1000°C for 1.5h, control NH 3 The flow rate is 80mL / min, and the product after vapor deposition is washed and dried. The specific surface area of the sample is 487m 2 / g.
Embodiment 3
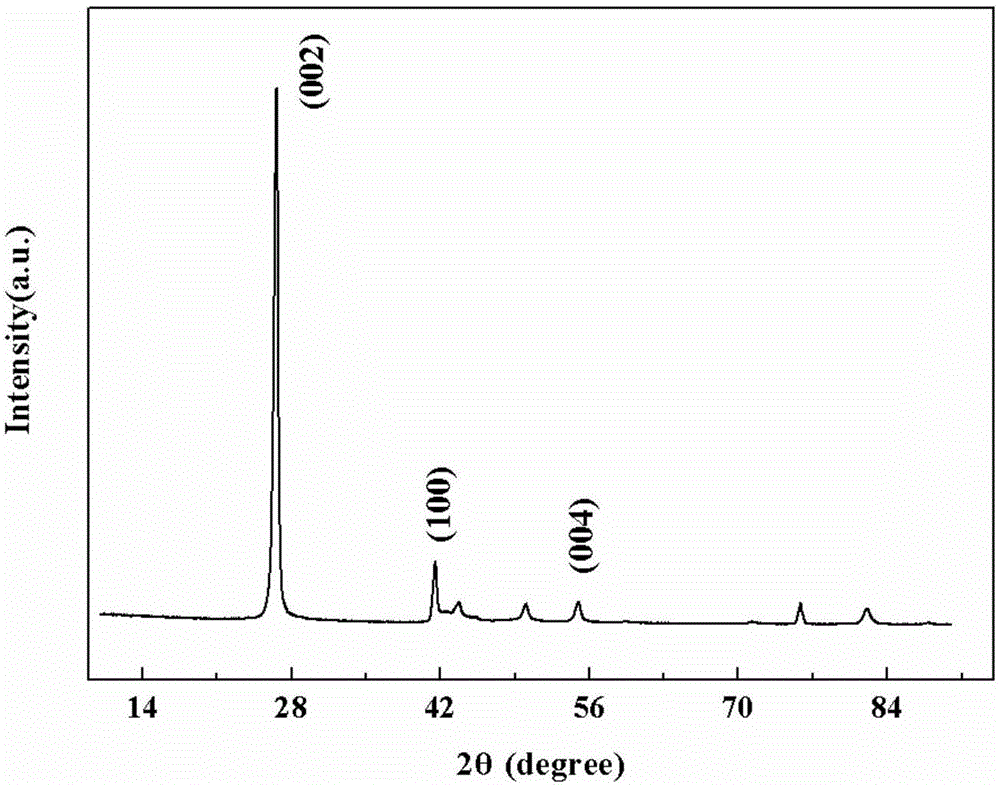
[0038] 0.33g of boron powder and 1.22g of magnesium oxide, the molar ratio of boron powder and magnesium oxide is 1:1, put into the ball mill jar and mill for 6 hours. The milled mixture was transferred to a BN boat in a vertical induction furnace. 200mL / min Ar purge and gradually increase the temperature, when it reaches 1300℃, introduce 100mL / min NH from bottom to top 3 , kept for 2 hours, the fluffy boron nitride was collected on the BN boat and the wall of the reaction chamber, and the product was characterized by SEM as a nanotube structure with a diameter ranging from a few nanometers to 70nm and a length of up to 10μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com