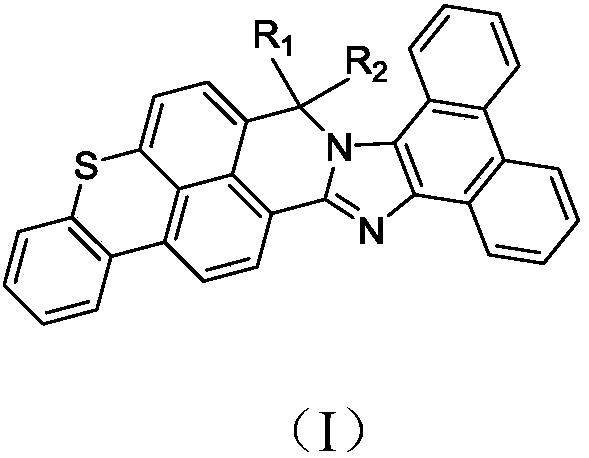
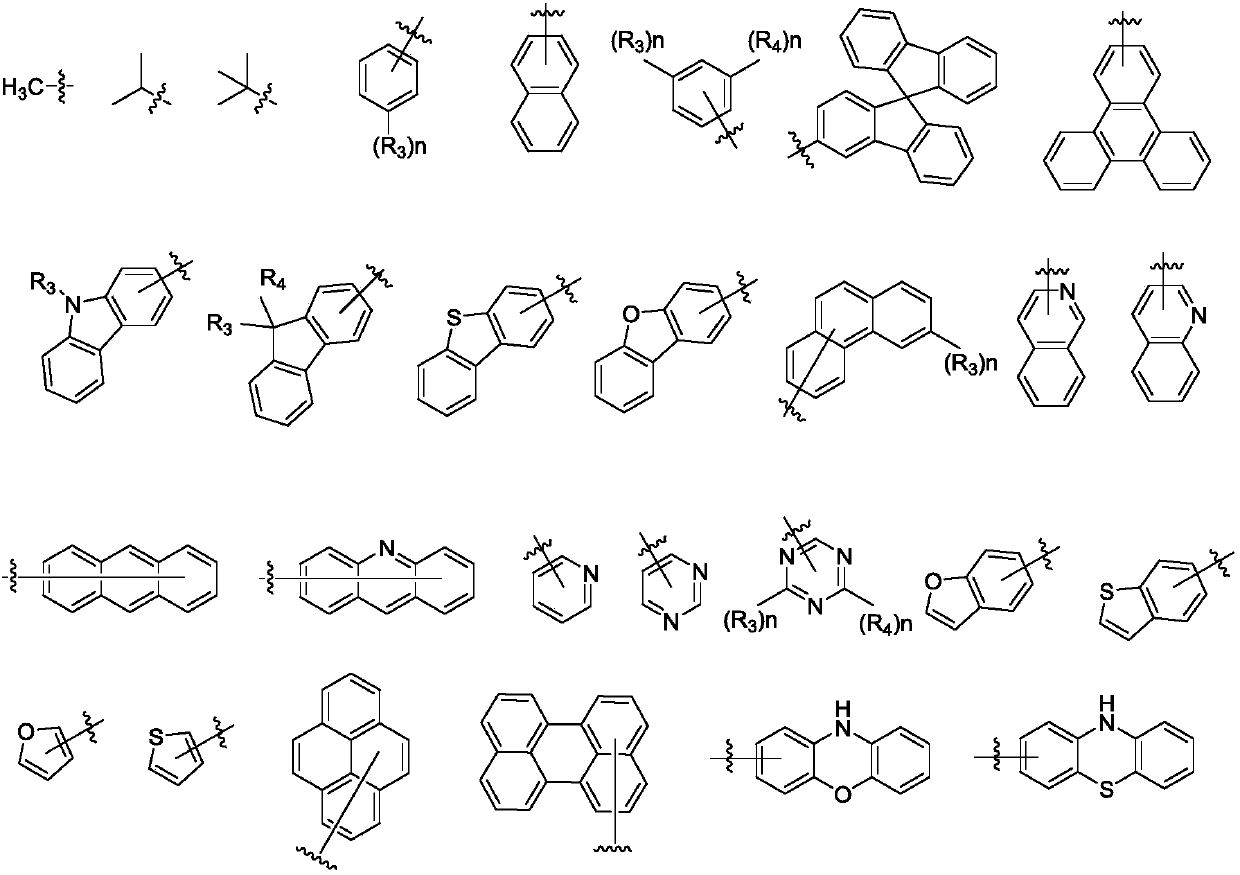
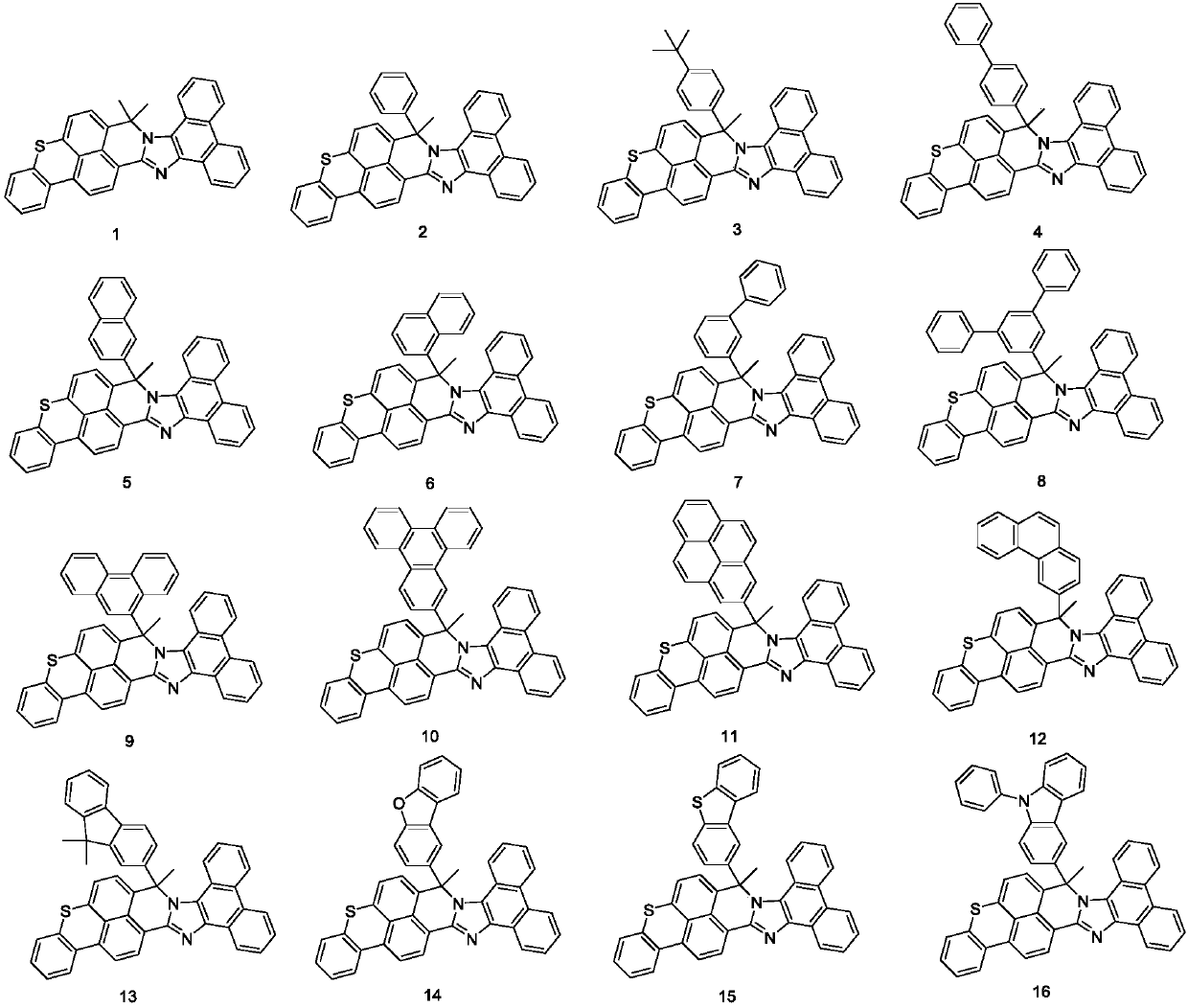
Derivative containing phenanthroimidazole structure and organic light-emitting device thereof
An organic light-emitting device, phenanthroimidazole technology, applied in the field of derivatives containing phenanthroimidazole structure and its organic light-emitting device, can solve the problems of low luminous efficiency, short device life, and OLED performance degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the synthesis of compound 1
[0052]
[0053] (1) In a four-neck flask equipped with a thermometer, a stirrer, a water separator and a condenser, add compound a (47mmol, 14.30g), compound phenanthrene 9,10 diamine (45mmol, 9.37g), ethylene glycol Diethyl ether (250ml) and pyridine (0.2g), stirred and heated to 140°C, kept the temperature for 3 hours, stirred and cooled the reaction solution to 40°C, filtered, washed the filter cake with ethanol, washed with water, and dried to obtain compound 1-1 .
[0054] (2) in N 2 Under protection, compound 1-1 (0.05mol, 23.84g) and 200ml of dry THF treated with Na / benzophenone were added to a 500ml three-neck flask, the reaction system was cooled to -40°C, and freshly prepared formazan was slowly added dropwise. Grignard reagent THF solution (prepared by methyl bromide (0.25mol, 23.73g) and magnesium (0.3mol, 7.2g)), after the dropwise addition was completed, the reaction system was slowly raised to room temperat...
Embodiment 2
[0058] Embodiment 2: the synthesis of compound 2
[0059] According to the synthetic method of compound 1, compound 2 (12.44 g, 75%) was obtained.
[0060]
[0061] Mass Spectrum m / z: 552.18 (calculated: 552.17). Theoretical element content (%)C 39 h 24 N 2 S: C, 84.75; H; 4.38; N, 5.07; S, 5.80 Measured element content (%): C, 84.76; H, 4.37; N, 5.07; S, 5.80. The above results confirmed that the obtained product was the target product.
Embodiment 3
[0062] Embodiment 3: the synthesis of compound 5
[0063] According to the synthetic method of compound 1, compound 5 (13.56 g, 75%) was obtained.
[0064]
[0065] Mass Spectrum m / z: 602.19 (calculated: 602.18). Theoretical element content (%)C 43 h 26 N 2 S: C, 85.69; H, 4.35; N, 4.65; S, 5.32 Measured element content (%): C, 85.69; H, 4.34; N, 4.66; S, 5.32. The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com