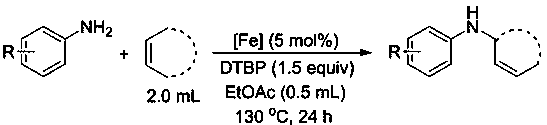Method for preparing allylamine compounds
An allylamine and compound technology, applied in the field of preparation of organic compounds, can solve problems such as narrow application range of substrates, and achieve the effects of clear catalyst structure, wide application range and easy air
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment one: containing 1,3-di-tert-butyl imidazolinium cation (molecular formula is [( t BuNCH 2 CH 2 N t Bu)CH][FeBr 4 ]) Synthesis of ionic iron complexes
[0029] Add 1,3-di-tert-butylimidazolinium chloride (0.22 g, 1.0 mmol) and NaBr (0.15 g, 1.5 mmol) sequentially to a solution of ferric tribromide (0.29 g, 1.0 mmol) in THF , reacted at 60 ° C for 24 hours, removed the solvent in vacuum, washed with hexane, drained, extracted with tetrahydrofuran, transferred the supernatant by centrifugation, added hexane to the supernatant for recrystallization, and precipitated reddish-brown crystals at room temperature with a yield of 90 %.
[0030] Carry out elemental analysis to product, the result is as follows:
[0031] Elemental analysis
[0032]
C:(%)
H:(%)
N:(%)
theoretical value
23.64
4.15
5.01
actual value
23.88
4.31
5.34
[0033] This complex [( t BuNCH 2 CH 2 N t Bu)CH][FeBr 4 ] exists in the for...
Embodiment 2
[0037] Embodiment two: [( t BuNCH 2 CH 2 N t Bu)CH][FeBr 4 ] catalyzed oxidative coupling reaction of aniline with cyclohexene
[0038] Add aniline (46 μl, 0.5 mmol), catalyst (14 mg, 0.025 mmol), di-tert-butyl peroxide (138 μl, 0.75 mmol) and cyclohexene (2 ml) to the reaction flask in sequence , ethyl acetate (0.5 ml) was reacted at 130°C for 24 hours, cooled to room temperature after the reaction, and the product was purified by column chromatography (using a mixed solvent of ethyl acetate / petroleum ether volume ratio of 1:50 as the developing solvent ), with a yield of 95%.
[0039] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 , TMS): 7.10-7.06 (m, 2H), 6.61-6.57 (m, 1H), 6.54-6.51 (m, 2H), 5.77-5.74 (m, 1H), 5.68-5.65 (m, 1H), 3.90 (s, 1H), 3.51 (s, 1H), 1.95-1.93 (m, 2H), 1.84-1.79 (m, 1H), 1.67-1.52 (m, 3H).
Embodiment 3
[0040] Embodiment three: [( t BuNCH 2 CH 2 N t Bu)CH][FeBr 4] catalyzed oxidative coupling reaction of p-methylaniline with cyclohexene
[0041] In the reaction flask, add p-methylaniline (54 mg, 0.5 mmol), catalyst (14 mg, 0.025 mmol), di-tert-butyl peroxide (138 microliters, 0.75 mmol), cyclohexene (2 ml), ethyl acetate (0.5 ml) was reacted at 120°C for 28 hours, cooled to room temperature after the reaction, and the product was purified by column chromatography, (with a mixed solvent of ethyl acetate / petroleum ether volume ratio of 1:20 For developing agent), the yield was 83%.
[0042] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 , TMS): 6.89 (d, J = 8.1 Hz, 2H), 6.46 (d, J = 8.4Hz, 2H), 5.76-5.72 (m, 1H), 5.69-5.64 (m, 1H), 3.87 (s, 1H), 3.26 (s, 1H),2.15 (s, 3H), 1.94-1.92 ( m, 2H), 1.81-1.78 (m, 1H), 1.66-1.50 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com